Malachite green in food
Abstract
Malachite green (MG) has been used globally in aquaculture but is not registered for use in food-producing animals in the European Union. The European Commission requested EFSA to evaluate whether a reference point for action (RPA) of 2 μg/kg for the sum of MG and its major metabolite leucomalachite green (LMG) is adequate to protect public health. Available occurrence data were not suitable for a reliable exposure assessment. The hypothetical dietary exposure was calculated, considering the RPA as occurrence value for all types of fish, fish products and crustaceans. Mean dietary exposure across different European dietary surveys and age classes would range from 0.1 to 5.0 ng/kg body weight (bw) per day. For high and frequent fish consumers, the exposure would range from 1.3 to 11.8 ng/kg bw per day. Both MG and LMG induced formation of DNA adducts in livers of rats and/or mice, and of micronuclei in mice. LMG also induced cII transgene mutations in mouse liver. MG caused a small, not dose-related, increase in thyroid gland follicular adenomas and carcinomas, and of mammary gland carcinomas in female rats. LMG caused an increase in hepatocellular adenomas and carcinomas in female mice. Both MG and LMG may be considered as carcinogenic and as genotoxic in vivo. A lower 95% confidence limit for a benchmark response of 10% extra risk (BMDL10) of 13 mg/kg bw per day for hepatocellular adenomas and carcinomas was selected as reference point for neoplastic effects. For non-neoplastic effects, a lower 95% confidence limit for a benchmark response of 5% extra risk (BMDL05) of 6 mg/kg bw per day was selected for the effect of MG on liver weight and of LMG on body weight. The margins of exposure were 1.1 × 106 or greater for neoplastic effects and 4.9 × 105 or greater for non-neoplastic effects. The CONTAM Panel concluded that it is unlikely that exposure to food contaminated with MG/LMG at or below the RPA of 2 μg/kg represents a health concern.
Summary
Malachite green (MG) has been used globally as a therapeutic agent in aquaculture, but it is not registered for use in food-producing animals in the European Union (EU). However, residues of MG and its primary metabolite leucomalachite green (LMG) have been detected in aquaculture products in monitoring programmes in EU Member States, indicating the need for continued surveillance of fish, fish products and crustaceans. Use of MG has not been reported in bivalve production, only in the farming of fish and crustaceans. A minimum required performance limit (MRPL) was established for analytical methods which were used to control the use of MG, being 2 μg/kg for the sum of MG and LMG in meat of aquaculture products. This MRPL is used as a reference point for action (RPA) by food control authorities.
RPAs should be low enough to exclude health risks for consumers. The EFSA Scientific Opinion, titled ‘Guidance on methodological principles and scientific methods to be taken into account when establishing Reference Points for Action (RPAs) for non-allowed pharmacologically active substances present in food of animal origin’, identified an approach for establishing RPAs for various categories of non-allowed pharmacologically active substances. Assuming that MG/LMG residues would only be present in fish and crustaceans, this guidance would lead to a so-called toxicologically based limit of quantification (TBLOQ) of 0.15 or 0.30 μg/kg food (for toddlers or adults, respectively) as the basis for the establishment of an RPA. This TBLOQ is below the current RPA of 2 μg/kg and lower than limits of quantification (LOQs) that are currently achievable by analytical methods used in routine monitoring. Therefore, a compound specific risk assessment is required and the European Commission (EC) asked the European Food Safety Authority (EFSA) to evaluate whether the existing RPA of 2 μg/kg for the sum of MG and LMG is adequate to protect public health.
Data on occurrence of MG/LMG in food were extracted from the EC database on monitoring of veterinary medicinal product residues and other substances in fish, fish products and crustaceans for the years 2002–2014. There were 548 targeted samples reported as non-compliant. Data from Norway for the years 2006–2014 were also considered. Data were also extracted from the Rapid Alert System for Food and Feed (RASFF) database for the years 2002–2014. There were 135 notification events reported for MG/LMG. The notifications covered the product categories fish and fish products, crustaceans and products thereof, farmed fish and products thereof (other than crustaceans and molluscs) and wild-caught fish and products thereof (other than crustaceans and molluscs).
The EFSA Panel on Contaminants in the Food Chain (CONTAM Panel) concluded that data extracted from the EC database and the RASFF database were not suitable to carry out a reliable human dietary exposure assessment. The CONTAM Panel calculated the hypothetical human dietary exposure considering as an occurrence value the RPA of 2 μg/kg for all types of fish, fish products and crustaceans. The mean hypothetical chronic dietary exposure across the different European dietary surveys and age classes, would range from a minimum of 0.1 ng/kg body weight (bw) per day for infants, elderly and very elderly to 5.0 ng/kg bw per day for toddlers. As there is a concern that high and frequent fish consumers might have elevated MG/LMG dietary exposure, the exposure for these consumers was considered separately. The 95th percentile hypothetical chronic dietary exposure in fish consumers only, across the different European dietary surveys and age classes, would range from a minimum of 1.3 ng/kg bw per day for adults to a maximum of 11.8 ng/kg bw per day for toddlers.
Concentrations of MG and LMG residues in fish muscle are reduced when the muscle is subjected to cooking conditions, such as boiling, baking and microwaving, or when stored under refrigeration, freezing or repeated freezing/thawing conditions. The concentrations of LMG, generally, are reduced to a lesser extent than those of MG.
There is scant information about MG and LMG kinetics in mammalian species and no data were identified for humans. In rats, MG is rapidly absorbed and excreted mainly by the faecal route. Available data indicate that MG undergoes biliary excretion, possibly as a glutathione (GSH) adduct. In orally dosed rats and mice, MG is reduced to LMG and both undergo hepatic sequential N-demethylation. An N-oxide derivative, resulting from the oxidative biotransformation of an N-demethylated metabolite, has been identified in liver extracts from LMG-administered mice.
In fish, MG is rapidly absorbed and extensively biotransformed to LMG, which is stored in tissues and slowly excreted. MG N-oxide and MG N-demethylated derivatives were also detected in edible fish tissues. Persistence of MG and LMG residues in fish depends on fish species and size, MG exposure concentration and duration, and environmental conditions including temperature and pH. In fish, MG was detected up to about 2 months and LMG was detected up to about 9 months after cessation of exposure. In crustaceans (shrimp), residues of MG/LMG were no longer detectable 9 days following treatment.
In 28-day toxicity tests, MG caused haematological effects in mice and rats, and an increase in liver weight in male and female rats. The overall no-observed-adverse-effect level (NOAEL), based on haematological effects in female rats, was 9.4 mg/kg bw per day. For LMG, in 28-day toxicity tests, an increase in liver weight was found in male rats. Hepatocyte vacuolisation was found at all doses tested, including the lowest dose of 30 mg/kg bw per day. Therefore, a NOAEL was not identified for LMG regarding hepatotoxicity.
For LMG, a NOAEL of 10 mg/kg bw per day for fetal toxicity in rats was identified. The CONTAM Panel noted, however, that these effects were observed at doses also causing maternal toxicity.
The positive results obtained in the in vivo micronucleus tests in mice for MG and LMG, the increased mutations in the cII transgene in mouse liver for LMG, and the capacity of both MG and LMG to form DNA adducts in vivo provide evidence for considering MG and LMG as genotoxic in vivo.
In long-term studies, an increase in liver weight was observed in male rats. LMG caused an increase in relative thyroid weight in male and female rats. MG was not carcinogenic in mice, but induced a small, not dose-related, increase in the incidence of thyroid gland follicular adenomas and carcinomas and of mammary gland carcinomas in female rats. The CONTAM Panel concluded that MG may be considered as carcinogenic. LMG caused an increase in hepatocellular adenomas and a small increase in hepatocellular carcinomas in mice. In rats, LMG caused a small increase in the incidence of mammary gland carcinomas and of thyroid gland follicular cell adenomas or carcinomas (combined). The CONTAM Panel concluded that LMG may be considered as carcinogenic.
For humans, only one report was identified, describing a case of methaemoglobinaemia in a 3-year-old girl following a single exposure to MG (about 2.6 mg/kg bw) in the form of an aquarium product containing 0.075% MG. However, the composition of the product was not reported.
Regarding the mode of action, MG has been shown to induce formation of reactive oxygen species (ROS) due to its behaviour as an electron-accepting/transferring compound. In addition, ROS can be formed from induction of cytochrome P450-dependent monooxygenases, as well as from oxidative biotransformation of MG itself. MG-mediated formation of ROS has been associated with MG-induced cytotoxicity, DNA damage, apoptosis, disturbances in cell cycle progression and mitogen-activated protein kinase signal transduction pathways and in vitro malignant transformation. In vivo, MG caused depletion of GSH and a decrease in the activity of glutathione-S-transferases and antioxidant enzymes that could lead to elevated levels of free radicals as indicated by increased hepatic lipid peroxidation. LMG has been shown to inhibit thyroid peroxidase (TPO) in vitro. It can also be biotransformed by TPO into N-demethylated derivatives that could, in turn, generate reactive metabolites. This may explain the effects observed in the thyroid.
Because MG and LMG may be regarded as substances that are genotoxic and carcinogenic, the derivation of a health-based guidance value is not appropriate. A lower 95% confidence limit for a benchmark response of 10% extra risk (BMDL10) for hepatocellular adenomas and carcinomas in female mice of 13 mg/kg bw per day was selected as a reference point for neoplastic effects of MG/LMG. Based on the effect of MG on liver weight and of LMG on body weight, the CONTAM Panel selected the lower 95% confidence limit for a benchmark response of 5% extra risk (BMDL05) of 6 mg/kg bw per day as a reference point for the non-neoplastic effects of MG/LMG.
Due to the limited occurrence data for MG/LMG, no reliable human dietary exposure assessment could be carried out and, therefore, the CONTAM Panel could not characterise the risk.
The CONTAM Panel evaluated whether an RPA of 2 μg/kg for the sum of MG and LMG is adequate to protect public health. For the average consumer, the median hypothetical chronic dietary exposure to MG/LMG across dietary surveys would result in margins of exposure (MOEs) for neoplastic effects of about 1.1 × 107 for toddlers and 2.2 × 107 for adults, and in MOEs for non-neoplastic effects of about 4.8 × 106 for toddlers and 9.7 × 106 for adults. For high and frequent fish consumers, the median hypothetical chronic dietary exposure to MG/LMG across dietary surveys would result in MOEs for neoplastic effects of about 2.1 × 106 for toddlers and 3.4 × 106 for adults, and in MOEs for non-neoplastic effects of about 9.5 × 105 for toddlers and 1.5 × 106 for adults. The CONTAM Panel considered that these MOEs for neoplastic and non-neoplastic effects are sufficiently large and do not indicate a health concern.
Overall, the CONTAM Panel concluded that it is unlikely that exposure to food contaminated with MG/LMG at or below the RPA of 2 μg/kg represents a health concern.
The CONTAM Panel recommends that more data should be generated on the occurrence of additional metabolites in fish and crustaceans. Further information on the fate of MG and LMG during food processing should be developed. In addition, knowledge concerning the toxicokinetics and bioavailability of MG/LMG using human in vitro models should be improved. Information regarding the potential genotoxicity of the N-demethylated metabolites should be developed, and the DNA adducts and GSH adducts observed in rodents should be characterised.
1 Introduction
1.1 Background and Terms of Reference as provided by the requestor
1.1.1 Background
Malachite green (MG; 4-{[4-(dimethylamino)phenyl](phenyl)methylidene}-N,N-dimethylcyclohexa-2,5-dien-1-iminium chloride) is a triphenylmethane dye. The term MG is the commonly used one. However, MG is also known as aniline green, basic green 4, diamond green B or Victoria green B. It is widely used to colour a diverse range of materials such as textile, leather and paper products. It also has several applications in diagnostic media.
MG has a longstanding history of use in aquaculture due to its antifungal and antiprotozoal infection activities in fish and fish eggs. Application dosages range from 0.15 mg/L for prolonged treatment in ponds to 100 mg/L for dipping solutions. In the European Union (EU), MG is not registered for use in food-producing animals. Although it is not an allowed substance for use in food-producing animals, residues of MG are regularly discovered in aquaculture products mainly imported from third countries, demonstrating the most likely illegal use triggered by its low cost, high efficacy, large availability and lack of alternatives.
MG treatment results in residues of MG and its metabolite leucomalachite green (LMG), both potential carcinogenic and/or genotoxic substances. In view of harmonising controls in the EU, a reference point for action (RPA) for the sum of residues of MG and LMG is applicable at 2 μg/kg. Based on information from the European Union reference laboratory (EURL)/National reference laboratory (NRL) network, the analytical performance could be slightly enhanced possibly realising detection at 1 μg/kg for the sum of MG and LMG, corresponding to the Reasonably Achievable Lowest Limit of Quantification in the ‘Guidance on methodological principles and scientific methods to be taken into account when establishing Reference Points for Action (RPAs) for non-allowed pharmacologically active substances present in food of animal origin’ (EFSA CONTAM Panel, 2013).
However, detection of residues of MG in wild-caught fish seems to indicate that MG is also an environmental contaminant. Possible routes of contamination of the environment could include: disposal of water containing MG used for treatment of ornamental fish, leaching of green colour from clothes during washing and industrial spills downstream of dye manufacturing plants. Levels of 0.765 μg/kg have been reported in such cases.
In a scenario where MG/LMG residues would only be present in fish and shellfish, the above-mentioned guidance considers a toxicologically based limit of quantification (TBLOQ) of 0.15 or 0.30 μg/kg food (based on toddler or adult exposure, respectively) as a basis for the establishment of an RPA (EFSA CONTAM Panel, 2013). As the TBLOQ is below the RPA of 2 μg/kg, a substance-specific risk assessment is required.
1.1.2 Findings
More than 60 notifications related to the presence of MG have been issued through the Rapid Alert System for Feed and Food (RASFF) over the last decade. All notifications concern fish and fish products and concern a wide variety of fish species, including fish eggs. Reported levels are usually below 20 μg/kg but exceptions up to 7,500 μg/kg have also been reported.
1.1.3 Terms of Reference
In view of a possible review of the existing RPA for MG/LMG and taking into account that the TBLOQ values are below what is currently analytically achievable and considering possible background contamination, the Commission requests a risk assessment as to whether the existing RPA of 2 μg/kg for the sum of MG and LMG is adequate to protect human health.
1.2 Interpretation of the Terms of Reference
The European Food Safety Authority (EFSA) received a request from the European Commission (EC) for a scientific opinion on the adequacy of the RPA for MG/LMG in food to protect human health.
- evaluation of the toxicity of MG and its metabolite LMG for humans, considering all relevant toxicological endpoints and identification of the toxicological relevance of MG and LMG present in food;
- exposure of the EU population to MG and LMG from food, including the consumption patterns of specific groups of the population such as high and frequent fish consumers;
- evaluation of whether the existing RPA of 2 μg/kg for the sum of MG and LMG is adequate to protect public health.
1.3 Additional information
1.3.1 Previous assessments
MG and its major metabolite LMG have been the subject of several previous assessments by international, European and national organisations.
1.3.1.1 International and European agencies
The EFSA Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC Panel) reviewed the toxicological information of a large number of dyes illegally present in food in the EU (EFSA, 2005b). MG and LMG were included in the group of ‘dyes that have been used illegally in countries outside the EU from which spices originate and dyes that have been used in the past as food colours in other countries but withdrawn from food use following discovery of toxicity’. The AFC Panel concluded that both compounds should be viewed as genotoxic and/or carcinogenic.
At its 70th meeting, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluated MG and LMG (FAO/WHO, 2009). In short-term (28 days) toxicity studies, MG caused haematological changes and liver toxicity in male and female rats with a no-observed-adverse-effect-level (NOAEL) of 10 mg/kg body weight (bw) per day and LMG induced liver effects in male rats at all doses tested (NTP, 2005). The increased incidence of fetal anomalies in all dose groups observed in a teratogenicity study in rabbits (Meyer and Jorgenson, 1983), although without a consistent dose–response relationship, raised concern regarding the potential developmental toxicity of MG. JECFA concluded, however, that this study was inadequately conducted and reported, and that additional studies are needed to evaluate the reproductive and developmental toxicity of MG.
JECFA concluded that MG has no genotoxic potential in conventional in vitro and in vivo assays. LMG was negative in in vitro assays, but induced cII mutations in the liver of female Big Blue® B6C3F1 transgenic mice, whereas MG did not. MG was not carcinogenic in a long-term study in female mice, but in female F344 rats a trend in the incidence of thyroid gland follicular cell adenomas or carcinomas was found. In a long-term study in female mice, LMG caused a dose-related increase in the combined incidence of hepatocellular adenomas and carcinomas. Because of the induction of cII mutations by LMG in liver cells of female transgenic mice, JECFA concluded that a genotoxic mechanism cannot be ruled out.
JECFA considered it inappropriate to establish an acceptable daily intake (ADI) for MG and used a margin of exposure (MOE) approach. Due to deficiencies in the database, it was considered inappropriate to derive an MOE for non-carcinogenic endpoints. It concluded that the induction of hepatocellular adenomas or carcinomas in female mice treated with LMG was the pivotal effect for the risk assessment, and derived a lower 95% confidence limit or a benchmark response of 10% extra risk (BMDL10) of 20 mg LMG/kg bw per day as the reference point for the MOE calculation. Because there was no information on the conversion rate of MG to LMG in food, JECFA considered it prudent to evaluate the sum of the residues of MG and LMG in food, expressed as LMG. Using average and high (97.5th percentile (P97.5)) exposures (see Section 3.2.1), MOEs of about 1.3 × 105 and 3 × 104, respectively, were calculated, which were considered to be of low concern for human health.
The European Chemicals Agency (ECHA) evaluated the same data on MG and LMG as used by JECFA (ECHA, 2010a,b). ECHA concluded that MG may cause developmental toxicity in rabbits, but noted that the poor quality of the study cast some doubts on the reliability of the findings. Based on the induction of mutations in the liver of transgenic mice by LMG and the induction of DNA adducts in the liver of rats and mice by MG and LMG, ECHA considered it prudent to presume that MG and LMG are potential in vivo somatic cell mutagens and, based on classification, labelling and packaging (CLP) criteria, a classification of ‘Muta.2, Suspected to cause genetic defects’ was proposed for both compounds.
In female rats, there were no significant increases in tumour incidence caused by MG, and no tumour findings in female B6C3F1 mice. ECHA considered the evidence as not sufficient to warrant a classification of MG for carcinogenicity. ECHA concluded that there was limited evidence for carcinogenicity of LMG based on tumour induction in the liver of mice and equivocal evidence of induction of tumours in the liver of female rats. Although it was recognised that the evidence for carcinogenicity is only weak, the possible involvement of a genotoxic action raised concerns for the carcinogenicity of LMG. Therefore, based on CLP criteria, a classification of ‘Carc.2, Suspected of causing cancer’ was proposed.
1.3.1.2 National agencies
The UK Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment (UK COM, 2004) and the UK Committee on the Carcinogenicity of Chemicals in Food, Consumer Products and the Environment (UK COC, 2004) evaluated MG and LMG. Based on the available conventional mutagenicity data, there was some evidence for a clastogenic potential of MG, but it was not possible to make an adequate assessment for LMG. However, due to the formation of DNA adducts in the liver from both rats and mice by MG, and the induction of mutations in liver DNA of female transgenic B6C3F1 mice by LMG, it was concluded that both compounds should be regarded as in vivo mutagens (UK COM, 2004). For MG, there was no convincing evidence for a carcinogenic effect in female F344 rats and female B6C3F1 mice, but there was equivocal evidence of carcinogenic activity of LMG in female F344 rats based on an increased incidence of hepatocellular adenomas or carcinomas (combined). Taking into account the views of the UK COM (2004), it was therefore considered prudent to regard LMG as a genotoxic carcinogen (UK COC, 2004).
The National Food Institute (NFI) of Denmark and the Technical University of Denmark (DTU) provided a risk assessment of MG in 2007 (NFI and DTU, 2007). It was reported that in the Danish surveillance programme up to 28 μg/kg of LMG was found in fish produced in Denmark. With an intake of 100 g fish/day, this led to a conservatively estimated exposure of 2.8 μg LMG/day (0.048 μg/kg bw per day for a 60-kg adult). Compared with a BMDL10 of 20 mg/kg bw per day for hepatocellular adenomas and carcinomas in female mice, this led to an MOE of more than 4 × 105, which would be a low concern for public health, and considered to be a low priority for risk management actions.
A health risk assessment of MG and LMG was reported by the Food Safety Commission (FSC) of Japan (FSC, 2005). Concerning genotoxicity it was concluded that the available in vitro and in vivo results ‘failed to provide a univocal explanation for various in vivo mutations including DNA adduct formation and cII mutation. Nevertheless, the results obtained so far could not deny the genotoxic potentials of MG and LMG. Further studies are required to reach a reliable conclusion’. According to the FSC, the results of the 2-year rodent studies suggested that MG acts as a weak carcinogen in the liver and mammary glands of female rats, and that LMG acts as a liver carcinogen in female mice and as a weak carcinogen in liver and thyroid of rats.
Food Standards Australia New Zealand (FSANZ) investigated the risk of exposure to MG in fish (FSANZ, 2007). Levels of MG in climbing perch of 7.8 μg/kg were found. Based on a mean fish consumption of 100 g/day, this resulted in a dietary exposure of about 0.011 μg/kg bw per day for a 70-kg adult. Based on the data on carcinogenicity and genotoxicity, it was suggested that ‘MG is a very low risk’, and the lowest-observed-effect level (LOEL) of 5 mg/kg bw for non-neoplastic lesions in the rat liver was considered as the most sensitive endpoint. The estimated dietary exposure was about 450,000 times below the LOEL, and it was therefore concluded that the health risk from MG residues in fish is extremely small.
In 2014, the Committee for Pharmacologically Active Substances and Veterinary Medicinal Products of the German Federal Institute for Risk Assessment (BfR) published a toxicological evaluation on MG (BfR, 2014). The Committee evaluated occurrence data on MG from official controls, analysis and toxicity of MG, the applicability of the threshold of toxicological concern (TTC) concept, an assessment based on the MOE and the appraisal by ECHA on MG. Based on a BMDL10 value of 20 mg LMG/kg bw for hepatocellular adenomas and carcinomas in female mice and an estimated human daily exposure of 5–50 ng LMG/kg bw, the Committee calculated MOEs of 4 × 106–4 × 105 and concluded that a daily exposure of 50 ng LMG/kg bw is toxicologically tolerable. In addition, the Committee evaluated the applicability of the TTC concept and concluded that MG and LMG fulfil the TTC criteria, which lead to a TTC value of 0.15 μg per day for MG/LMG. In summary, the Committee stated that MG should not be administered in food-producing animals and recommended to apply the TTC value of 0.15 μg per day for the health assessment of MG/LMG residues in food as this represents the more conservative approach compared to other toxicological assessments, in particular, when taking sensitive population groups into account.
The CONTAM Panel noted the risk assessment by l'Agence française de sécurité sanitaire des aliments (AFSSA; currently ANSES (Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail)) from 2002 (AFSSA, 2002). However, since the bulk of information regarding carcinogenicity of MG/LMG became available after 2002, no further information regarding this risk assessment is provided in this Scientific Opinion.
1.3.1.3 Other assessments
Rauscher-Gabernig et al. (2007) assessed the risk to the Austrian population from exposure to MG and LMG. Fish samples, mainly fresh fish produced in Austria, collected in the period 2003–2005, contained residues almost entirely of MG. The estimated intake for high fish consumers (95th percentile, P95), expressed as LMG, was 0.83 μg/kg bw per day for children (3–6 years of age), 0.42 μg/kg bw per day for adult women and 0.51 μg/kg bw per day for adult men. Applying a BMDL10 of 21 mg/kg bw per day for liver tumours in female mice and thyroid tumours in male rats, these intakes resulted in MOEs of 2.5 × 104, 5 × 104 and 4.1 × 104 for these three age groups, respectively. Therefore, the authors classified the health risk to the Austrian population from exposure to MG and LMG as low.
Based on the maximum concentration of LMG found in wild eel caught in Germany (see Section 3.1.1) and chronic fish consumption data, intakes of 0.27 and 3.8 ng/kg bw per day for children and adults, respectively, were reported by Schuetze et al. (2008). Using a LOEL of 13 mg LMG/kg bw per day for the increased incidence of neoplasms (NTP, 2005), the authors reported MOEs of 4.9 × 107 and 3.4 × 106 for children and adults, respectively.
The application of the MOE approach to substances in food that are genotoxic and carcinogenic was studied by a group of international scientists (Benford et al., 2010). LMG was one of the 12 compounds addressed. Available literature data suggested that only about 10–15% of fish contained residues of LMG, and data generally did not distinguish between farmed and wild-caught fish. Average and high (P95) dietary intakes were estimated to be 5 and 50 ng/kg bw per day, respectively. The authors considered these intake estimates to be highly conservative because it had been assumed that all fish consumed was contaminated at the mean LMG concentration reported in surveillance studies. Based on a BMDL10 of 20.4 mg/kg bw per day for liver tumours (adenomas and carcinomas combined), the MOE for average and high consumers were 4 × 106 and 4 × 105, respectively. Therefore, it was concluded that LMG is likely to be a relatively low priority for risk management actions, unless specific incidents result in higher levels of exposure. Identical MOEs for LMG were reported by Renwick et al. (2010).
The risk of human exposure to LMG due to fish consumption in Taiwan was assessed by Chu et al. (2013). They conducted a probabilistic risk assessment for three population groups, adolescents (13–18 years), adults (19–64 years) and elderly (> 64 years), with elderly consumers having the highest intake of LMG. Based on a BMDL10 of 18.5 mg/kg bw per day for hepatocellular adenomas and carcinomas in female mice and probabilistic estimates of the lifetime average daily dose for mean and high (P95) adult consumers, MOEs for these consumers were 4.8 × 106 and 4.1 × 105, respectively. Using a cancer slope factor of 0.035 mg/kg bw per day, cancer risk estimates for this age group were 4.7 × 10−7 at a mean and 1.6 × 10−6 at a 95th percentile lifetime average daily dose. The authors concluded that daily intake of LMG from fish consumption in Taiwan was of low priority for risk assessment, except for those with 95th percentile high intake. The CONTAM Panel identified some inconsistencies in the way the results were reported, and noted that had the reported point estimates for exposure of average (13.5 ng/kg bw per day) and high adult consumers (45 ng/kg bw per day) been used, MOEs of 1.4 × 106 and 4.1 × 105, respectively, would have resulted. However, these results do not affect the conclusions of Chu et al. (2013).
1.3.2 Therapeutic use of malachite green in fish
Antibiotics and fungicides are used in aquaculture to prevent or treat fish diseases, which are often caused by stress conditions (such as high fish density, hypoxia, and high nitrite and ammonia concentrations), which impair the immune system and consequently increase susceptibility to infection. MG was historically one of the most frequently used disinfectants in fish farming (particularly of salmonids), and its fungicidal properties have been known since the 1930s (Foster and Woodbury, 1936). In the 1950s, MG was used as an antiseptic against both internal and external parasites. In the 1960s, MG proved to provide the most effective treatment against protozoan ectoparasites, particularly Ichthyophthirius multifiliis. The use of MG became even more widespread when it was shown to be effective against aquatic fungi Saprolegnia spp. in fish eggs (Olah and Farkas, 1978; Alderman and Polglase, 1984) and for the treatment of proliferative kidney disease of salmonids (Clifton-Hadley and Alderman, 1987).
MG has previously been used globally as a therapeutic agent in aquaculture, particularly in fish and crustacean farming. For example, it was found to be the most frequently used therapeutic product at 78 freshwater fish farms examined in Germany by Manz et al. (1991). MG has primarily been administered via aquatic exposure either as a flush treatment at a concentration of 1 mg/L or as a bath treatment over a longer period at a lower concentration (e.g. 6 days at 0.2 mg/L for rainbow trout; Máchová et al., 1996 and 6 days at 0.5 mg/L for cyprinids; Sudova et al., 2007). MG has also been used as a fungicide in fishmeal, and consequently been demonstrated to be present in commercial fish feed (Conti et al., 2015).
MG is not registered for use in food-producing animals in the EU (see Section 1.3.5). However, MG/LMG residues in aquaculture products have been detected in monitoring programmes in EU Member States (Verdon et al., 2015), indicating its continued use in certain geographic areas and the need for continued surveillance of fish products. Use of MG has not been reported in bivalve culture, only in fish and crustacean farming.
1.3.3 Chemistry
1.3.3.1 Malachite green
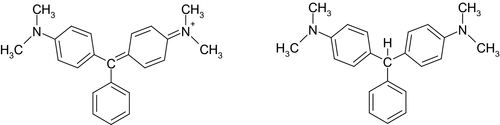
MG1 ([4-[[4-(dimethylamino)phenyl]-phenylmethylidene]cyclohexa-2,5-dien-1-ylidene]-dimethylazanium;chloride; Chemical Abstract Service (CAS) No 569-64-2) is a cationic dye and consists of green crystals with metallic lustre. It has the molecular formula C23H25N2Cl and a molecular weight of 364.91 g/mol. The oxalate salt (CAS No 2437-29-8, molecular weight 463.50 g/mol) is also marketed. The green colour of the compound is independent of whether it occurs with the chloride or oxalate anion.
The melting point is 158–160°C. When heated to decomposition, it emits toxic fumes of nitrogen oxide and hydrogen chloride. MG occurs almost entirely in the ionised form at pH values of 5–9. It has a good solubility in water and alcohols. MG is easily converted into LMG (see Section 3.3.1).
1.3.3.2 Leucomalachite green
LMG1 (4-[[4-(dimethylamino)phenyl]-phenylmethyl]-N,N-dimethylaniline; CAS No 129-73-7) consists of an off-white to light brown powder. From alcohol and benzene, it forms needles or leaflets. It has the molecular formula C23H26N2 and a molecular weight of 330.46 g/mol (Figure 1).
The melting point is 102°C. In contrast to MG, the estimated solubility of LMG in water, being 6.4 × 10−2 mg/L at 25°C, is very low (EPI (estimation program interface) Suite, estimated). LMG is very soluble in organic solvents, such as ethanol, ethyl ether, ethylene glycol monomethyl ether and benzene.
1.3.4 Analytical methods
1.3.4.1 Sampling and storage
Most of the sampling of food, and of related materials, for MG/LMG testing in foods of animal origin is undertaken in the context of the national residue monitoring plans as specified in Council Directive 96/23/EC,2 with residue testing undertaken in accordance with Commission Decision 2002/657/EC3. For details of the protocols and procedures specified for such sampling and testing, see Section 2.1.1 of this opinion.
Commission Decision 2002/657/EC states that samples shall be obtained, handled and processed in such a way that there is a maximum chance of detecting the substance. Sample handling procedures shall prevent the possibility of accidental contamination or loss of analytes. To achieve this goal, samples are stored in suitable, secure, clearly identified containers and in conditions, such as frozen storage (fish and crustaceans, water) or at refrigerated/ambient temperatures (fish feed), prior to analysis.
1.3.4.2 Specific issues relating to (leuco)malachite green analysis
Initially, testing for residues of MG in animal tissues was by methods directed at the parent compound, using high-performance liquid chromatography (HPLC) with ultraviolet/visible (UV/Vis) detection. However, studies on MG showed that residues of the parent compound are less persistent than those of the reduced metabolite LMG. Therefore, subsequent HPLC methods measured both substances using a combination of UV/Vis detection (618 nm) for MG and fluorescence detection (λex 265 nm/λem 360 nm) for LMG, or measurement of both substances as MG following oxidation of LMG to MG. MG and LMG are the targets for residue analysis in food, while testing for the parent compound alone is limited to samples of fish feed and water.
Because MG and LMG are sensitive to air and light, particular steps need to be taken to protect both analytical standards and sample residues from deterioration. Addition of ascorbic acid to standard solutions of LMG, to prevent photo-oxidative demethylation, and of acetic acid to standard solutions of MG, to prevent transformation of MG to its carbinol form, has been applied (Hall et al., 2008; Ahn et al., 2010) and standard solutions are normally stored in amber bottles (Mitrowska et al., 2008a; Hurtaud-Pessel et al., 2011). In the case of sample treatment, protection from light is recommended and steps, such as addition of hydroxylamine hydrochloride as a reducing agent/antioxidant (Hurtaud-Pessel et al., 2011) or use of acidified acetonitrile to reduce conversion of MG to its carbinol form (Hall et al., 2008) have been applied.
1.3.4.3 Extraction and sample clean-up
Extraction of MG/LMG from fish and crustacean samples is most often carried out using mixtures of low pH buffer (McIlvane pH 3 or ammonium acetate buffers) and organic solvent (acetonitrile), but also using direct extraction with organic solvents such as acetonitrile. Typically, the solvent extract is subjected to liquid/liquid partitioning with dichloromethane or defatting by washing with hexane, and further clean-up is achieved by the addition of adsorbents, such as alumina, basic alumina or primary/secondary amines, to the extract or by using solid-phase extraction (SPE). For SPE, a range of sorbents have been applied including reversed phase (such as C18 and polymeric sorbents), normal phase (such as alumina sorbents) and cation exchange (such as propylsulfonic acid sorbents). Depending on whether the method is a screening or confirmatory method, less or more sample extract purification steps may be required.
Other approaches have been applied to extraction/clean-up of MG/LMG from fish and crustacean samples, such as the Quick Easy Cheap Effective Rugged Safe (QuEChERS) technique, molecularly imprinted polymers (MIPs) and immunoaffinity chromatography (IAC). The QuEChERS, or dispersive SPE, technique involves use of a combination of salts and SPE sorbents to achieve a combined extraction and clean-up of MG/LMG from the sample matrix. IAC involves use of MG specific antibodies immobilised on a support material, following oxidation of residues of LMG to MG with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), to isolate MG/LMG from sample extracts (Xie et al., 2013). MIPs involve use of an imprinted polymer with selectivity for both MG and LMG (Long et al., 2009) or MG specific imprinted polymers with pre-MIP oxidation of LMG to MG using DDQ (Martínez Bueno et al., 2010; Guo et al., 2011); the MIP is packed into a column to trap the analyte(s) from the sample extract and allow for washing steps prior to elution of the analyte(s) from the column. In a variation on the MIPs approach, Huang et al. (2015) describe use of a magnetic MIP to selectively enrich MG/LMG from fish sample extracts.
In the case of water samples, some screening methods based on determination by enzyme-linked immunosorbent assay (ELISA) (Yang et al., 2007; Xing et al., 2009) or conductive carbon black paste electrode (Qu et al., 2012) use simple dilution of the filtered sample. Other methods use solvent extraction (Maleki et al., 2012), microwave-assisted ionic liquid extraction (Gao et al., 2013), hollow fibre liquid-phase microextraction (Zou et al., 2014), cloud point extraction (Pourreza and Elhami, 2007; An et al., 2010), direct SPE (Safarik and Safarikova, 2002; Mitrowska et al., 2008a) or MIP-based SPE to isolate MG alone (Lian and Wang, 2012) or MG and LMG (Li et al., 2008) from water samples.
Extraction of MG from fish feed samples was undertaken by solvent extraction with acetonitrile and clean-up of the extract by MIP-based SPE (Li et al., 2008).
1.3.4.4 Screening methods
Screening methods should measure MG/LMG with sufficient sensitivity to satisfy regulatory requirements, currently at the minimum required performance limit (MRPL) of 2.0 μg/kg for the sum of MG and LMG in meat of aquaculture products (Annex II of Commission Decision 2002/657/EC). Screening methods for MG/LMG include immunoassays (ELISAs, RNA aptamer assay, biosensors), electrochemiluminescence and HPLC.
A number of ELISAs have been developed for determination of LMG (Singh et al., 2011) and for both MG and LMG (Yang et al., 2007; Xing et al., 2009; Xu et al., 2013) with limits of detection (LOD) between 0.05 and 0.5 μg/kg in fish. Zhang et al. (2015) describe a chemiluminescent enzyme immunoassay for MG with a limit of quantification (LOQ) of 0.1 μg/kg in fish and shrimp. Commercial ELISA kits are available for determination of both MG and LMG with reported LOD values of 0.1–0.25 μg/kg in fish and crustaceans.
As an alternative to antibodies used as the binding agents in conventional ELISAs, use of an RNA-aptamer has been described by Stead et al. (2010); this method was capable of determining both MG and LMG (following oxidation to MG) in salmon tissue at levels considerably lower than the MRPL of 2 μg/kg.
Biosensor-based assays have been described, such as an electrochemical method for determination of MG using a multiwall carbon nanotube-modified glassy carbon electrode (Yi et al., 2008) and an electrochemical enzyme inhibition sensor on a screen-printed carbon working electrode for determination of MG and LMG in fish, with LODs of 0.25 μg/kg (Faridah et al., 2013; Hidayah et al., 2013).
Methods based on determination by electrochemiluminescence have been described: one using molecularly imprinted SPE for isolation of MG with an LOQ of 0.02 μg/kg (Guo et al., 2011) and another using magnetic MIPs to isolate MG and LMG with an LOQ of 0.036 μg/kg (Huang et al., 2015).
HPLC methods for determination of MG and LMG in fish and crustacean samples may involve direct measurement of both substances using UV/Vis (618–620 nm) and fluorescence (λex 265 nm/λem 360 nm) detectors, respectively; Mitrowska et al. (2005) describe a method for carp with decision limits (CCα4) of 0.15 and 0.13 μg/kg for MG and LMG, respectively, and detection capability (CCβ5) values of 0.37 and 0.32 μg/kg for MG and LMG, respectively, while Chen and Miao (2010) describe a method for catfish with LOD values of 0.38 and 0.10 μg/kg for MG and LMG, respectively. Where oxidation of LMG to MG is performed, such oxidation may be undertaken pre-HPLC with DDQ or post-column with lead oxide (PbO2) or with iodine. A number of methods using pre-HPLC derivatisation with DDQ have been described for salmon, shrimp and catfish with LOD values of 0.15–1.0 μg/kg (Andersen et al., 2005, 2006, 2009), for fish with an LOD value of 0.15 μg/kg (Xie et al., 2013) and for trout with CCα/CCβ values of 0.16/0.39 μg/kg (Fallah and Barani, 2014). Methods involving post-column derivatisation with PbO2 have been described for catfish (Roybal et al., 1995) and for catfish and trout (Rushing and Thompson, 1997) with LOQ values of 0.5–1.5 μg/kg, for fish and prawns with an LOD value of 1.0 μg/kg (Bergwerff and Scherpenisse, 2003; Stoev and Stoyanov, 2007), and for fish, shrimp and shellfish with CCα/CCβ values of 0.14/0.24 μg/kg (Long et al., 2009). Long et al. (2008) describe an alternative post-column oxidation procedure for LMG using an iodine solution, giving CCα/CCβ values of 0.17/0.23 μg/kg.
Most HPLC methods for water samples test only for MG with UV/Vis detection (600–618 nm) and reported LOD values between 0.01 and 0.1 μg/L (Li et al., 2008; Lian and Wang, 2012; Maleki et al., 2012; Gao et al., 2013; Zou et al., 2014). A method for determination of MG (CCα/CCβ values of 0.03/0.08 μg/L) and LMG (CCα/CCβ values of 0.03/0.07 μg/L) in water samples using UV/Vis and fluorescence detectors was described by Mitrowska et al. (2008a).
A number of methods have been described for the determination of MG, used as a dye, in meat (Sun et al., 2013), various coloured foods (Dixit et al., 2011) and medicinal herbs (Li et al., 2015). These methods involved microwave-assisted extraction with SPE clean-up and HPLC with UV/Vis detection, solvent extraction with SPE clean-up and HPLC with UV/Vis detection, and use of a silver nanoparticle wiper with surface-enhanced Raman scattering detection, respectively.
1.3.4.5 Confirmatory methods
Liquid chromatography–mass spectrometry (LC–MS) is the method of choice for confirmatory analysis of MG/LMG in fish and crustaceans. Of the various mass spectrometric methods available, liquid chromatography linked with a triple quadrupole mass detector is most commonly used, although ion trap (Turnipseed et al., 2005; Wu et al., 2007; Andersen et al., 2009; Martínez Bueno et al., 2010) and time-of-flight (Villar-Pulido et al., 2011) detectors have also been used. Some of the published methods for MG/LMG are multiresidue methods, also including other triphenylmethane dyes, such as (leuco)crystal violet and/or (leuco)brilliant green (Dowling et al., 2007; Wu et al., 2007; Tarbin et al., 2008; Andersen et al., 2009; Chen and Miao, 2010; Hurtaud-Pessel et al., 2011, 2013; Tao et al., 2011). A method using time-of-flight-mass spectrometry (TOF-MS) has been developed for multiclass determination of residues of selected pharmacologically active substances, including MG/LMG, in shrimps (Villar-Pulido et al., 2011).
Typical MS conditions used for the confirmatory analysis of MG/LMG are positive electrospray ionisation (ESI) with two precursor to product ion transitions being monitored for each analyte, m/z 329 > 313, 208 for MG and m/z 331 > 239, 316 for LMG. Sample treatment, prior to liquid chromatography–tandem mass spectrometry (LC–MS/MS) determination of the analytes, typically involves buffer/organic solvent extraction coupled with clean-up by SPE, but extraction by automated solvent extraction (Tao et al., 2011) or by QuEChERS (Villar-Pulido et al., 2011; Hashimoto et al., 2012; Lopez-Gutierrez et al., 2013) and clean-up using MIPs (Martínez Bueno et al., 2010) have also been reported.
LC–ESI–MS/MS methods for determination of MG/LMG have been applied to a variety of fish, including catfish, trout, salmon and tilapia, and to shrimps. The range of values for CCα and CCβ by these methods, for MG and for LMG, are < 0.1–0.9 and 0.1–1.2 μg/kg, respectively (van de Riet et al., 2005; Dowling et al., 2007; Halme et al., 2007; Arroyo et al., 2009; Chen and Miao, 2010; Hurtaud-Pessel et al., 2011, 2013; Tao et al., 2011; Ascari et al., 2012; Hashimoto et al., 2012; Xu et al., 2012; Lopez-Gutierrez et al., 2013; Andersen et al., 2015); all with method sensitivity sufficient to satisfy the MRPL value of 2 μg/kg. A number of papers have been published on the determination of total MG in fish, following oxidation of any LMG present in the sample to MG, post-column using PbO2 with reported CCα/CCβ values of 0.11/0.15 μg/kg (Scherpenisse and Bergwerff, 2005) and pre-LC using DDQ with reported CCα/CCβ values of 1.2/2.0 μg/kg (Tarbin et al., 2008).
LC–MS methods using an ion-trap detector have been developed for analysis of total MG in salmon and catfish, following oxidation of any LMG present in the sample to MG using DDQ, with reported LOD/LOQ values of < 0.15/0.15 μg/kg (Turnipseed et al., 2005), 0.24/0.75 μg/kg (Andersen et al., 2009) and 0.003/0.005 μg/kg (Martínez Bueno et al., 2010). Isotope dilution liquid chromatography/mass spectrometry (ID-LC/MS), using deuterated and/or 13C6-MG and -LMG, has been developed for fish, shrimp and shellfish (Doerge et al., 1998; Wu et al., 2007; Ahn et al., 2010). LOD values of 0.02 μg/kg for MG and of 0.5 μg/kg for LMG are reported (Doerge et al., 1998; Ahn et al., 2010) and CCα/CCβ values of 0.08/0.13 μg/kg for MG and of 0.05/0.09 μg/kg for LMG are reported (Wu et al., 2007). An accurate mass full-scan TOF-MS method has been applied to the analysis of shrimp, with reported LOD values of 0.06 and 0.60 μg/kg for MG and LMG, respectively (Villar-Pulido et al., 2011).
An LC–ESI–MS/MS method has been developed for MG/LMG in water samples, following centrifugation and preconcentration on diol SPE columns, with reported CCα/CCβ values of 0.04/0.06 μg/L for MG and of 0.03/0.05 μg/L for LMG (Mitrowska et al., 2008a).
1.3.4.6 Analytical quality assurance: performance criteria, reference materials and proficiency testing
The performance criteria for methods used to test for MG/LMG are those laid down in Commission Decision 2002/657/EC for screening and confirmatory methods. Methods must have satisfactory performance for the characteristics of specificity, trueness, ruggedness, and stability of the analyte in standard solutions and in test matrices. The methods must be validated for recovery, repeatability, within-laboratory reproducibility, calibration curves, CCα and CCβ according to procedures specified in the Decision, or equivalent procedures.
MG oxalate (94.3 ± 1.4% purity) and LMG (98.8 ± 0.8% purity) reference standards have been prepared and are commercially available (Le Goff and Wood, 2008). Isotopically labelled materials, such as d5-MG, d5- or d6-LMG and 13C6-LMG, are available commercially for use as internal standards. No certified reference materials for MG/LMG are commercially available to date. A sample of salmon to be used in an international intercomparison study was produced and the sum of MG and LMG in this sample was determined to be 9.32 ± 0.98 μg/kg (at the 95% confidence interval) using ID-LC/MS (Hall et al., 2008).
Several proficiency tests and interlaboratory studies have been reported for MG/LMG in fish. The EURL, Anses-Fougères (France), provides proficiency testing for National Reference Laboratories in charge of control for dye residues in aquaculture products with three rounds having been organised covering trout (2005, 2009) and prawn (2013) samples (Verdon et al., 2015). In January 2008, the Proficiency Test Advisory Board, Government Laboratory, Hong Kong reported on a proficiency test (APLAC T058) aimed at evaluating the testing capability of 48 participating laboratories in the quantitative analysis of MG and LMG in swamp eels; 48% and 62% of participants had satisfactory results (z-score ≤ 2) for the determination of MG (assigned value 28.2 μg/kg) and LMG (assigned value 2.21 μg/kg), respectively (Wong, 2008). In the UK, the Food Analysis Performance Assessment Scheme (FAPAS) provides samples of fish muscle containing MG and LMG for testing.6
1.3.4.7 Concluding comments
While a combination of buffer/solvent extraction, liquid/liquid partitioning and SPE is the most commonly used sample preparation for the analysis of MG/LMG using both screening and confirmatory methods, other approaches, such as the QuEChERS technique, MIPs and IAC have been applied. Screening methods for MG/LMG include HPLC with UV/Vis and fluorescence detectors, ELISA and biosensor methods and electrochemiluminescence, all providing sufficient analytical sensitivity to meet the MRPL of 2 μg/kg. Confirmatory methods are based on various LC–MS methods and these typically provide CCα and CCβ values of < 1.0 μg/kg for a range of fish and crustaceans, again adequately meeting the MRPL of 2 μg/kg.
1.3.5 Legislation
According7 to Article 3 of Regulation (EC) No 470/20098, any pharmacologically active substance intended for use in the EU in veterinary medicinal products (VMPs) which are to be administered to food-producing animals shall be subject to an opinion of the European Medicines Agency (EMA) on the maximum residue limit (MRL), formulated by the Committee for Medicinal Products for Veterinary Use (CVMP). The opinion consists of a scientific risk assessment and risk management recommendations. Pharmacologically active substances, for which the opinion concludes that no MRL is needed or that a (provisional) MRL should be established, are subsequently classified in Table 1, ‘allowed substances’, of Regulation (EU) 37/20109. All use of other pharmacologically active substances in VMPs is not allowed. A specific group of the non-allowed substances is the group of ‘prohibited substances’, listed in Table 2 of Regulation (EU) 37/2010. MG is not listed in Table 1 of Regulation (EU) 37/2010. Therefore, although not explicitly listed in Table 2, MG is not registered and not allowed for use in food-producing animals in the EU.
Article 18 of Regulation (EC) No 470/2009 stipulates that, for substances which are not classified as ‘allowed substances’ in accordance with that Regulation, an RPA may be established to ensure the functioning of controls for food of animal origin. Food of animal origin containing residues of such substances at or above the RPA is considered not to comply with EU legislation. Until now, RPAs have been based only on analytically driven MRPLs, and no consideration has been given to the toxicological profile of non-allowed substances. An MRPL of 2 μg/kg for meat of aquaculture products is specified in Annex II of Commission Decision 2002/657/EC3, which was amended by Commission Decision 2004/25/EC10. This MRPL refers to the sum of MG and its metabolite LMG.
Under the terms of Commission Decision 2005/34/EC11, the MRPLs listed in Annex II of Commission Decision 2002/657/EC are currently to be used as RPAs, irrespective of the matrix tested, for the purpose of the control of residues when analytical tests are being carried out in the framework of import control. However, this Decision regulated imports from third countries only and did not apply to food produced within the Union. As a number of animal products originating from Member States were found to contain non-allowed and prohibited substances below and above the MRPLs, the EC and the Member States agreed to also apply the approach laid down in Decision 2005/34/EC, with the necessary changes, to food of animal origin produced within the Union. This implies, in particular, that the MRPLs set in accordance with Commission Decision 2002/657/EC shall also be used as RPAs. This approach, moreover, means that any detection of substances, the use of which is not authorised in the Union, regardless of the level found, shall be followed by an investigation into the source of the substance in question and appropriate enforcement measures shall be applied, in particular, aiming to prevent reoccurrence in the case of documented illegal use (SANCO-E.2(04)D/521927).12
2 Data and methodologies
2.1 Data
2.1.1 Occurrence data
Data on the occurrence of MG/LMG in food are not currently collected by EFSA. The only analytical results on MG/LMG present in the EFSA Chemical Occurrence database have been submitted by the Czech Republic (80 samples). The Czech Republic confirmed that the same data were also submitted to the EC database on residues of veterinary medicines, relating to the national residue monitoring plan (see below).
2.1.1.1 National residue monitoring plans
Council Directive 96/23/EC on measures to monitor certain substances and residues thereof in live animals and animal products requires that Member States draft a national residue monitoring plan for the groups of substances detailed in Annex I of this Directive. These plans must comply with the sampling rules in Annex IV of the Directive.
Within Group B, Veterinary drugs and contaminants, of Annex I, Subgroup B3e Dyes, covering MG/LMG, samples are to be taken only from aquaculture animals, their feeding stuffs, water and primary animal products. The minimum number of samples to be collected each year is specified as a proportion of the aquaculture production volume of the previous year. The compounds sought and the samples selected for analysis should be selected according to the likely use of these substances. Sampling under the national residue monitoring plan should be targeted; samples should be taken preferably at the farm, on fish ready to be placed on the market, and either at the processing plant or at wholesale level. Samples taken at farm level should be taken from a minimum of 10% of registered sites of production.
Member States submit data on the occurrence of non-compliant results determined in the residue monitoring, including for MG/LMG to the EC database on residues of veterinary medicines. Data on the occurrence of MG/LMG in food have been extracted from the EC database on residues of veterinary medicines. This database contains the annual sampling plan and the results from 2004 onwards provided by all Member States. The results are reported as aggregate data. However, there is no indication of the sample matrix tested and no concentration for the chemical residue or contaminant detected in the sample is provided. In addition, the number of samples analysed for the individual substances are reported by the Member States only if there is at least one non-compliant sample for the substance in question. Where all samples are compliant, the number of samples analysed is not reported. Furthermore, where controls are carried out at farm and processing plant, the total number of samples recorded may refer to samples taken at either farm or processing plant, depending on where the non-compliant samples were found, and this may be on a substance group basis rather than on the individual substance basis. Where non-compliant samples were found at both farm and processing plant, the number of samples represents the sum of samples taken at both sampling points.
For the years 2002 and 2003, data on MG/LMG reported by Member States have been extracted from the Commission Staff Working Papers on the implementation of national residue monitoring plans in the Member States (EC, 2004, 2005). The data presented in these papers are not at the same level of detail as found on the database for the years 2004–2014. For example, for 2003 the number of samples analysed represents the total number of targeted samples tested for all categories of substances, rather than the number of samples specifically tested for Subgroup B3e Dyes.
Data on the occurrence of MG/LMG in food have been considered for the period 2002–2014.13
In addition to the data submitted to the EC database on residues of veterinary medicines from Member States, data from Norway on the occurrence of MG/LMG for the years 2006–2014 were also considered. The latter were extracted from the annual reports ‘Monitoring program for residues of therapeutic agents, illegal substances, pollutants and other undesirables in farmed fish (in accordance with Council directive 96/23/EC)’ (NIFES, 2007, 2008, 2009, 2010, 2011, 2012, 2013, 2014, 2015).
2.1.1.2 Rapid Alert System for Food and Feed
The CONTAM Panel considered the Rapid Alert System for Food and Feed (RASFF)14 database as another source of information on the occurrence of MG/LMG in food.
RASFF notifications mostly concern controls at the outer European Economic Area (EEA) borders, at points of entry or border inspection posts, when a consignment is not accepted for import into the EU. The second largest category of notifications concerns official controls on the internal market. A small number of notifications may be triggered by official controls in non-member countries.
After an inspection is conducted within a country and unfavourable results of the analysis are obtained, the risk needs to be evaluated, as does the probability that the product may be present on the market of other Member States. In the latter case, notifications are provided when non-compliant samples for a contaminant are found, providing also quantified values. However, information on the total number of samples analysed, the number of compliant samples, the concentrations and the type of analysis undertaken is rarely provided.
Searches in the RASFF database were performed for the hazard category ‘residues of veterinary medicinal products’ – hazard ‘malachite green’ or ‘leucomalachite green’ – that had been notified between 1 January 2002 and 31 December 2014.
2.1.2 Consumption data
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database) provides a compilation of existing national information on food consumption at individual level. It was first built in 2010 (EFSA, 2011a; Huybrechts et al., 2011; Merten et al., 2011). Details on how the Comprehensive Database is used are published in the Guidance of EFSA (EFSA, 2011a).
The latest version of the Comprehensive Database15 contains results from a total of 51 different dietary surveys carried out in 23 different Member States covering 94,532 individuals.
- Infants: < 12 months old
- Toddlers: ≥ 12 months to < 36 months old
- Other children: ≥ 36 months to < 10 years old
- Adolescents: ≥ 10 years to < 18 years old
- Adults: ≥ 18 years to < 65 years old
- Elderly: ≥ 65 to < 75 years old
- Very elderly: ≥ 75 years old
Overall, the food consumption data gathered by EFSA in the Comprehensive Database are the most complete and detailed data currently available in the EU. Consumption data were collected using single or repeated 24- or 48-h dietary recalls or dietary records covering from 3 to 7 days per subject. Owing to the differences in the methods used for data collection, direct country-to-country comparisons can be misleading.
2.1.3 Food classification
Consumption data were classified according to the FoodEx classification system (EFSA, 2011b). FoodEx is a food classification system developed by EFSA in 2009 with the objective of simplifying the linkage between occurrence and food consumption data when assessing the exposure to hazardous substances. It contains 20 main food groups (first level), which are further divided into subgroups having 140 items at the second level, 1,261 items at the third level and reaching about 1,800 endpoints (food names or generic food names) at the fourth level.
In 2011, a new version of FoodEx, named FoodEx2 has been developed and is described in the scientific document ‘Report on the development of a Food Classification and Description System for exposure assessment and guidance on its implementation and use’ (EFSA, 2011c). The last release of FoodEx2 complements the previous hierarchical classification system of basic codes with more detailed food levels and gives the possibility of reporting additional information through the use of facets and facet descriptors (EFSA, 2015).
2.1.4 Toxicokinetic and toxicological data
All data were obtained from the scientific literature as described in Section 2.2.2.
2.2 Methodologies
2.2.1 Dietary exposure assessment in humans
The CONTAM Panel considered that only chronic dietary exposure to MG had to be assessed. As suggested by the EFSA Working Group on Food Consumption and Exposure (EFSA, 2011a), dietary surveys with only 1 day per subject were not considered as they are not adequate to assess repeated exposure. Similarly, subjects who participated only 1 day in the dietary studies, when the protocol prescribed more reporting days per individual, were also excluded for the chronic exposure assessment. Thus, for chronic exposure assessment, food consumption data were available from 35 different and most recent dietary surveys carried out in 19 different European countries present in the latest version of the Comprehensive Database (Appendix B).
For calculating chronic dietary exposure to MG/LMG, food consumption and body weight data at the individual level were accessed in the Comprehensive Database. Occurrence data (in this particular case the RPA value of 2 μg/kg) and consumption data were linked at the lowest FoodEx level possible. For each dietary survey, exposure estimates were calculated per age class. It should be noted that not all countries provided consumption information for all age groups, and in some cases, the same country provided more than one consumption survey. The mean and the high (95th percentile) chronic dietary exposures were calculated by combining for all types of fish, fish products and crustaceans (excluding aquatic molluscs) the RPA value with the average daily consumption for each food at individual level in each dietary survey.
To calculate a more refined dietary exposure to MG/LMG, the CONTAM Panel considered that the above-mentioned foods are also consumed as part of composite dishes present in the FoodEx classification system. These are, for example, fish sauce, fish-based meals, soups and salads. In addition, in the case of infants and toddlers, fish is normally consumed through ‘Ready-to-eat meals for infants and young children’. Therefore, where it was clearly stated in the name of the meal that this was a mixture of fish and other food items (e.g. fish and rice meal, fish and potatoes meal, etc.), a factor of 0.5 was applied, meaning that half of the quantity reported to be consumed was considered as referring to fish and the other half to the other food items present in the dish. All composite dishes and fish sauce were grouped as ‘Fish composite dishes’. In the case of ‘Ready-to-eat meals for children’, the only FoodEx code description that refers to fish is the ‘Ready-to-eat meal for children, meat/fish-based’. Considering that all foods classified under this code description would lead to an overestimation of exposure to MG/LMG, the CONTAM Panel referred either to the FoodEx2 code, where available, or the original food description to isolate only the fish-based meals and thus refine the exposure calculation.
The chronic dietary exposure to MG/LMG for high and frequent consumers of fish was calculated taking into consideration only subjects that consumed fish among the whole population from 35 different and most recent dietary surveys carried out in 19 different European countries present in the latest version of the Comprehensive Database.
All analyses were run using the SAS Statistical Software (SAS enterprise guide 5.1).
2.2.2 Literature search and appraisal of studies
2.2.2.1 Strategy for literature search
A comprehensive search for literature was conducted for peer-reviewed original research pertaining to adverse health effects on (experimental) animals and humans. The search strategy was designed to identify scientific literature dealing with toxicity, mode of action, toxicokinetics and human data on MG and LMG. An overview of the search terms is given in Appendix A, Section A.1.
It should be noted that a narrative approach was used for those sections dealing with methods of analysis, chemistry, occurrence and exposure as the identified papers are used only to give background information to the reader. For methods of analysis and chemistry, recent reviews, in combination with a limited literature search, were used to identify the scientific literature (see Appendix A, Section A.1.). Occurrence data were only considered when the samples had been collected since 2002, because the Member States became aware of the possible misuse of MG in aquaculture in 2002 and so control of MG was intensified by increasing the number of samples taken (EC, 2004).
The literature search was not restricted to publications in English. A first literature search was performed in April 2015 for all topics (October 2015 for papers on the occurrence in foods due to illegal use of MG as a food colouring agent). The literature search has been updated in December 2015 to identify papers dealing with toxicity, mode of action, toxicokinetics and human data on MG and LMG. Web of Science16 and PubMed17 were identified as databases appropriate for retrieving literature for the present evaluation. The references resulting from the literature search were imported and saved using a software package (EndNote18), which allows effective management of references and citations. Additionally, reviews, relevant scientific evaluations and toxicity studies by national or international bodies were considered for the current risk assessment, i.e. AFSSA (2002), NTP (2004, 2005), UK COM (2004), UK COC (2004), FSC (2005), (NFI and DTU, 2007), FSANZ (2007), FAO/WHO (2009), ECHA (2010a,b), and the BfR (2014). In addition, when relevant papers were identified during the risk assessment process (e.g. from other studies or reviews), they were also considered.
The references obtained were screened using title and abstract to identify relevant literature. The exclusion criteria used are shown in Appendix A, Section A.2.
2.2.2.2 Appraisal of studies
The information retrieved has been reviewed by the CONTAM Standing Working Group (SWG) on non-allowed pharmacologically active substances in food and feed and their RPAs, and has been used for the present assessment based on expert judgement. Any limitations in the information used are documented in this scientific opinion.
Selection of the scientific papers for inclusion or exclusion was based on consideration of the extent to which the study was relevant to the assessment and on general study quality considerations (e.g. sufficient details on the methodology, performance and outcome of the study, on dosing and route of administration and on statistical description of the results (EFSA, 2009b)), irrespective of whether they yielded positive, negative or null results.
Studies solely focusing on the efficacy of MG as a pharmacologically active substance were excluded from the assessment.
2.2.3 Methodology applied for risk assessment
The CONTAM Panel applied the general principles of the risk assessment process for chemicals in food as described by WHO (2009), which include hazard identification and characterisation, exposure assessment and risk characterisation. In addition to the principles described by WHO (2009), EFSA guidance pertaining to risk assessment has been applied for this assessment. For details on the specific EFSA guidance applied, see Appendix A, Section A.3.
3 Assessment
3.1 Occurrence data
3.1.1 Previously reported occurrence data
According to the Commission Staff Working Paper, in 2002 Member States became aware of the possible misuse of MG in aquaculture and so control of MG was intensified by increasing the number of samples taken (EC, 2004). Therefore, the CONTAM Panel considered occurrence data for samples that have been collected since 2002. It should be noted that some of the studies described in this section may also be included in the databases described in Section 3.1.2, relating to samples taken in national residue monitoring plans. The information presented below provides examples of the occurrence of MG and LMG in foods.
3.1.1.1 Fish and crustaceans
Bergwerff and Scherpenisse (2003) analysed trout (n = 18), eel (n = 10) and salmon products (n = 20) collected in the Netherlands for the presence of MG/LMG residues using HPLC-UV (LOD: 1 μg/kg) or LC–MS/MS (LOD: 0.2 μg/kg). The year of sampling was not indicated. LMG was detected in 13 trout samples (range: 1.3–14.9 μg/kg), five eel samples (range: 1.5–9.7 μg/kg), three samples of fresh salmon (range: 0.2–2.9 μg/kg), two smoked salmon samples (0.2 μg/kg in both samples) and in none of the canned salmon samples.
Schuetze et al. (2008) analysed wild eel (n = 45; year of sampling not indicated) caught from lakes, a river and a canal in Berlin, Germany. MG residues (reported as the sum of MG and LMG) were detected in 25 samples at concentrations ranging from 0.053 to 0.765 μg/kg. The authors stated that the occurrence could be linked directly to the presence of treated sewage discharge in the surface waters from which the fish was caught. Samples were analysed using LC–MS/MS (LOD/LOQ: 0.02/0.04 μg/kg for MG and 0.01/0.02 μg/kg for LMG).
Samples of carp (n = 42) and rainbow trout (n = 30), collected between 2009 and 2011 from fish farms in Croatia, were analysed for MG residues using an ELISA specific for MG (LOD/CCβ: 0.31/0.68 μg/kg). MG was detected in 13 samples but at concentrations below the MRPL of 2 μg/kg (Bilandžić et al., 2012).
Conti et al. (2015) analysed samples of sea bass (n = 15) and gilthead sea bream (n = 15) collected from an aquaculture plant in Italy in 2013. An ELISA method with an LOD/LOQ of 0.2/0.5 μg/kg was used. MG was detected at concentrations up to 1.21 μg/kg (mean concentration: 0.48 μg/kg).
Samples of wild European eel were collected between 2000 and 2009 in Belgium (n = 91 sites; 1 eel/site). Samples were analysed using ultra-performance liquid chromatography (UPLC)–MS/MS (LOD/LOQ: 0.25/0.05 μg/kg). LMG and MG were detected in 74.8% and 25.3% of the samples, respectively. Concentrations up to 9.61 and 0.96 μg/kg (mean concentrations: 0.56 and 0.07 μg/kg) were reported for LMG and MG, respectively (Belpaire et al., 2015).
In Australia, a national survey was conducted in April–June 2005 for which 60 samples of local and imported farmed finfish were analysed. LMG was detected in 10 samples and in two of these MG was also detected. The highest concentration detected was 880 μg/kg for LMG in fish imported from Vietnam. Samples were analysed using LC–MS/MS (LOQ: 2 μg/kg) (FSANZ, 2005).
In the framework of a Canadian total diet study, 12 composite samples of marine, freshwater and canned fish and shrimp were collected between 2002 and 2004. MG and LMG were analysed using LC–MS/MS (LOD: 0.15 μg/kg for both MG and LMG). LMG was detected in two composite samples of freshwater fish and one composite shrimp sample (0.95, 0.73 and 1.2 μg/kg) (Tittlemier et al., 2007).
Lee et al. (2010) reported on the analysis of 253 processed fish products collected from local markets in Korea (year of sampling not indicated). Samples were analysed using HPLC-diode array detection (DAD) (LOQ: 1.0 μg/kg) and positive results were confirmed by LC–MS/MS. MG/LMG residues were detected in one shrimp sample at a concentration of 2.6 μg/kg.
Samples of catfish nuggets (n = 24) were analysed in the USA for the presence of MG and LMG (year of sampling not reported). ELISA was used as the screening method (LOD: 1 μg/kg for the sum of MG, LMG, crystal violet and leucocrystal violet, using antibodies specific for MG and crystal violet following oxidation of the leuco metabolites). MG/LMG was not detected in any of the samples (Ozbay et al., 2013). The same method was also used to analyse tilapia fillets imported into the USA (n = 36; year of sampling not reported), but none of the samples tested positive for MG or LMG (Babu and Ozbay, 2013).
Samples (n = 144) of rainbow trout, collected in 2011 from fish farms in Iran, were analysed for the presence of MG/LMG using HPLC-DAD (CCα/CCβ: 0.16/0.39 μg/kg for MG). MG residues (sum of MG and LMG) were detected in 49% of the samples at concentrations ranging from 0.3 to 146.1 μg/kg and in 33% of the samples at the level of the MRPL or higher (Fallah and Barani, 2014).
3.1.1.2 Use of malachite green as a colouring agent
In 2005, the AFC Panel reviewed the toxicology of some dyes and MG was considered in the group of ‘dyes that have been used illegally in countries outside the EU from which spices originate and dyes that have been used in the past as food colours in other countries but withdrawn from food use following discovery of toxicity’ (EFSA, 2005b). Also in the scientific literature, MG has been reported to occur as a colouring agent in food.
Ashok et al. (2014) used micellar LC-DAD (LOD/LOQ: 100/250 μg/kg) to analyse samples of green peas (n = 8), ice candy (n = 5) and chili sauce (n = 5) that had been collected in India (year of sampling not reported). MG was detected in six samples of green peas (concentration range: 2,350–4,490 μg/kg), three samples of ice candy (concentration range: 2,410–4,290 μg/kg) and one sample of chili sauce (concentration: 250 μg/kg). Similar results were reported for samples collected in India before 2002 (Tripathi et al., 2007).
Dixit et al. (2011) collected samples of candy floss, sweetened puffed rice, cream biscuits, fruit cakes, coloured fried peas, sugar-coated fennel, cereal/pulse-based sweets, sugar toys and starch-based savoury products including fryums (n = 5 for each product) from a local market in India (year of sampling not reported). Analysis was done using HPLC-UV/Vis (LOD/LOQ: 0.195–0.695/0.62–2.21 mg/L or mg/kg). MG was detected together with auramine in one sample of fryums (concentration of the sum: 65,560 μg/kg). The same authors reported the analysis of 2,409 samples of milk-based sweets, non-milk/cereal-based sweets and savoury items collected in India (year of sampling not reported; Dixit et al., 2013). MG was detected in 11 samples (0.46%) at concentrations ranging from 57,400 to 231,000 μg/kg (median: 128,500 μg/kg).
The occurrence of MG in foods available on the EU market, due to use of MG as a food colouring agent, has not been reported. The CONTAM Panel noted the illegal use of MG as a colouring agent in samples from different foodstuffs collected in India. However, the data pertaining to foods in India are not considered relevant for the current risk assessment.
3.1.2 Current occurrence data
3.1.2.1 National residue monitoring plans
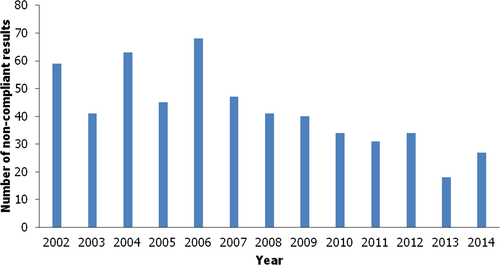
In the period 2002–2014, at least 21,00019 targeted samples of aquaculture products (ranging from at least 860–2,600 per year) were analysed for Subgroup B3e Dyes in all Member States and Norway.20 For MG/LMG, 548 targeted samples were reported to be non-compliant (levels not reported, see Section 2.1.1). The non-compliant samples were distributed across the years as shown in Figure 2. The highest number of non-compliant samples was reported in 2006. An explanation of the limitations of the data is given in Section 2.1.1.
3.1.2.2 Rapid Alert System for Food and Feed
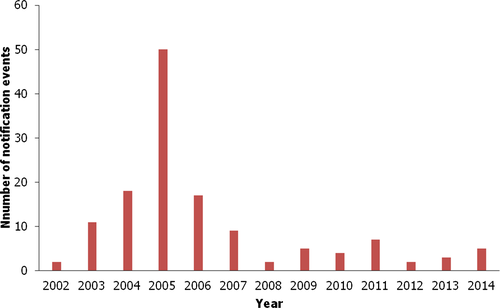
In the RASFF database, there were 135 notification events21 reported for MG/LMG in food products for the period 2002–2014, as seen in Figure 3. The notifications covered the following product categories: fish and fish products, crustaceans and products thereof, farmed fish and products thereof (other than crustaceans and molluscs)22 and wild-caught fish and products thereof (other than crustaceans and molluscs).23 The highest number of notifications (50) was reported in 2005.
3.1.3 Food processing
A study was undertaken to investigate the effects of cooking, including boiling, baking and microwaving, on residues of MG and LMG in carp muscle (Mitrowska et al., 2007). Following exposure of the fish in a bath containing a solution of 2 mg/L MG for 3 h, the fish were euthanised and the muscles collected. The muscle sample from the treated fish was mixed with blank material to obtain average concentrations of 209.8 μg/kg MG and 142.1 μg/kg LMG. HPLC with UV and fluorescence detection was used to determine the levels of MG and LMG in the samples.
Boiling (100°C) and baking (180°C) for 15 min reduced the level of MG in carp muscle by 54%, whereas LMG showed no measurable losses under these conditions. Microwave cooking of carp muscle for 1 min reduced the level of MG by 61% and the level of LMG by 40%. The authors concluded that the study suggests that MG/LMG residues in fish tissue may only be partly destroyed by high-temperature cooking.
Another study was undertaken to investigate the effects of cooking, including boiling and microwaving, on residues of MG in Oreochromis niloticus, tilapia (Farag et al., 2012). Of 150 samples taken from fish farms, MG residues were detected in 28 samples at concentrations ranging from 2.4 to 35.5 μg/kg, with a mean value of 11.8 μg/kg. Aliquots of the homogenised fish muscle samples were subjected to boiling for 15 min or to microwave cooking for 1 min. The mean concentrations of MG determined in the treated samples were 5.6 and 4.7 μg/kg following treatment by boiling or microwave cooking, respectively. These represent a reduction by 52.5% and 60.0% following thermal processing of the tilapia samples; measurable levels of MG were determined in all 28 samples after processing.
Salmon samples spiked with 35 μg/kg MG and 50 μg/kg LMG were heated at 70°C for 20 min; the recoveries of MG and LMG following this treatment were 29 ± 2% and 81 ± 1%, respectively. An incurred sample, containing 2.9 μg/kg LMG, subjected to the same treatment showed a concentration of 1.9 ± 0.3 μg/kg, representing a recovery of 65.5% after treatment (Bergwerff and Scherpenisse, 2003).
The stability of MG and LMG in rainbow trout samples stored at refrigeration and freezer temperatures was studied (Bergwerff and Scherpenisse, 2003). Rainbow trout samples spiked with 35 μg/kg MG and 50 μg/kg LMG were stored at 4°C for up to 4 days; a reduction of approximately 40% in MG and LMG was observed. Rainbow trout samples spiked with 50 μg/kg MG and LMG and with 500 μg/kg MG and LMG were stored at −20°C for up to 6 months; a reduction of less than 20% MG and of approximately 10% LMG was observed. The effect of three freeze–thaw cycles on stability of MG and LMG in rainbow trout (spiked at 35 μg/kg and 50 μg/kg, respectively) was studied and reductions of 24% MG and 16% LMG were observed.
In conclusion, the results of these studies indicate that the concentrations of MG residues in fish muscle are reduced by 50% or more when subjected to normal cooking conditions, such as boiling, baking and microwaving. The concentrations of LMG residues in fish muscle are also reduced under these cooking conditions, but to a lesser extent. Reduction in the concentrations of MG and LMG residues in fish muscle was also observed under refrigeration, freezing and repeated freezing/thawing conditions; again, the reduction of MG residues was greater, generally, than that of LMG residues.
3.2 Dietary exposure assessment for malachite green in humans
3.2.1 Previously reported exposure assessments in humans
Based on data collected in the UK between 1995 and 2006, showing mean and P97.5 levels of 30.7 and 138 μg/kg fish for the sum of MG and LMG (expressed as LMG), JECFA (FAO/WHO, 2009) estimated exposure levels of 150 and 690 ng/kg bw per day for the average and high (P97.5) intake (assuming a portion size of 300 g for an adult of 60 kg). A very similar range of exposure was estimated by Rauscher-Gabernig et al. (2007) for high fish consumers in the Austrian population, being 830 ng/kg bw per day for children (3–6 years of age), 420 ng/kg bw per day for adult women and 510 ng/kg bw per day for adult men. A much lower exposure of 0.27 and 3.8 ng/kg bw per day was reported for children and adults by Schuetze et al. (2008). Also, Benford et al. (2010) arrived at a much lower exposure of 5 (average) and 50 (P95) ng/kg bw per day, even though this was considered to be a highly conservative estimate. Similar exposure was estimated by Chu et al. (2013), who performed a probabilistic exposure assessment for the Taiwanese population and arrived at intake levels of 13.5 and 45.1 ng/kg bw per day for the average and high (P95) consumer. FSANZ calculated the exposure based on one MG level in climbing perch and arrived at an intake of 11 ng/kg bw for an adult consuming 100 g of this fish (FSANZ, 2007). The NFI of Denmark and DTU made an assessment on the highest observed level of 28 μg/kg fish, resulting in a highest exposure of 48 ng/kg bw per day for a 60-kg person consuming 100 g fish (NFI and DTU, 2007).
3.2.2 Current exposure assessment in humans
The CONTAM Panel noted the illegal use of MG as a colouring agent in samples from different foodstuffs collected in India (see Section 3.1.1). However, in Europe the occurrence of MG in foods due to use of MG as a food colouring agent has not been reported to date. The occurrence of MG as a colouring agent in food is therefore not included in the current exposure assessment. As the use of MG in food-producing animals is limited to fish and crustaceans, the CONTAM Panel considers it unlikely that MG occurs in other foods of animal origin and the exposure assessment is therefore limited to fish and crustaceans.
Only limited occurrence data on MG and LMG in food were available for this opinion (see Section 2.1.1 for an explanation of the limitations of the data). Therefore, the CONTAM Panel concluded that these data are not suitable to carry out a reliable human dietary exposure assessment.
The CONTAM Panel calculated the hypothetical human chronic dietary exposure using the RPA value of 2 μg/kg for all types of fish, fish products and crustaceans (excluding aquatic molluscs), and the results are presented in Table 1. Therefore, it should be noted that all reference to ‘dietary exposure’ in this and the following section is not based on actual occurrence data but refers to the hypothetical chronic dietary exposure based on the RPA value.
The mean dietary exposure and the high dietary exposure (P95) to MG/LMG were calculated separately for each survey and age class using consumption data recorded at individual level from the Comprehensive Database (see Section 2.1.2). In accordance with the specification of the EFSA Guidance on the use of the Comprehensive Database (EFSA, 2011a), 95th percentile estimates for dietary surveys/age classes with fewer than 60 observations may not be statistically robust and therefore are not considered in the risk characterisation.
The median MG/LMG dietary exposure for average consumers varied between 0.4 ng/kg bw per day in the infants group and 1.2 ng/kg bw per day in the toddlers group, across dietary surveys. The median dietary exposure for high consumers ranged from 1.4 ng/kg bw per day in the infants group to 5.5 ng/kg bw per day in the toddlers group. The highest maximum dietary exposure for average consumers was seen in the toddlers group, although the highest maximum dietary exposure for high consumers was seen in the other children group.
The CONTAM Panel also noted that, for the other children group, the difference between the maximum for the mean and for the 95th percentile dietary exposure is substantial. This can be explained by taking into consideration the different consumption levels of fish and fish products among the two age groups, as well as the range of ages covered by each of them. In the toddlers group (12 months –< 36 months old), there is not a big difference between consumption at the mean and 95th percentile as the consumption of fish and fish products is relatively low in the age range covered by this group. On the contrary, the other children group covers a wider age range (36 months to < 10 years old) and this is why different consumption levels of fish and fish products may occur between, for example, a 3- and a 9-year-old subject.
| Age class | Number of surveys | Hypothetical chronic dietary exposure | ||
|---|---|---|---|---|
| Minimum | Median | Maximum | ||
| Mean | ||||
| Infants | 6 | 0.1d | 0.4 | 1.1 |
| Toddlers | 10 | 0.5 | 1.2 | 5.0 |
| Other children | 18 | 0.3 | 1.1 | 2.7 |
| Adolescents | 17 | 0.2 | 0.6 | 1.5 |
| Adults | 17 | 0.2 | 0.6 | 1.9 |
| Elderly | 14 | 0.1 | 0.7 | 1.9 |
| Very elderly | 12 | 0.1 | 0.7 | 1.8 |
| 95th percentile c | ||||
| Infants | 5 | 0.0d | 1.4 | 5.8 |
| Toddlers | 7 | 3.3 | 5.5 | 6.2 |
| Other children | 18 | 2.3 | 4.7 | 9.1 |
| Adolescents | 17 | 1.6 | 2.7 | 5.7 |
| Adults | 17 | 1.6 | 3.0 | 4.9 |
| Elderly | 14 | 1.2 | 2.9 | 5.2 |
| Very elderly | 9 | 1.1 | 2.7 | 3.6 |
- bw: body weight.
- To avoid the impression of too high a precision being used, the numbers for all exposure estimates are rounded to two figures.
- a The minimum, median and maximum of the mean and 95th percentile exposure values across dietary surveys in European countries are shown.
- b This scenario contains all type of fish, fish products and crustaceans (excluding aquatic molluscs) that are contaminated with malachite green/leucomalachite green at a concentration equal to the reference point for action of 2 μg/kg.
- c The 95th percentile estimates obtained from dietary surveys/age classes with fewer than 60 observations may not be statistically robust (EFSA, 2011a) and therefore are not included in this table.
- d The lower minimum for the 95th percentile exposure in comparison to the minimum for the mean exposure is due to a very low number of infants (< 5%) consuming a high amount of fish in one survey resulting in the mean being higher than the 95th percentile in that survey.
The dietary exposure to MG/LMG was also calculated for pregnant and lactating women based on food consumption data originating from two surveys, one for each population group. The mean dietary exposure estimates (0.6 ng/kg bw per day for pregnant women and 1.0 ng/kg bw per day for lactating women) as well as the 95th percentile dietary exposure estimates (3.0 ng/kg bw per day for pregnant women and 3.2 ng/kg bw per day for lactating women) were similar to the respective mean and high (P95) exposures for the adults group.
3.2.3 Dietary exposure to MG/LMG for specific groups
3.2.3.1 High and frequent fish consumers
There is a concern that high and frequent consumers of fish might have elevated levels of MG/LMG dietary exposure. To test such a hypothesis, the 95th percentile hypothetical chronic dietary exposure from the daily consumption of fish among ‘consumers only’ was retrieved from the Comprehensive Database for surveys in which the number of selected participants exceeded 60.
The minimum, median and maximum of the 95th percentile MG/LMG dietary exposures for fish consumers are shown in Table 2. The dietary exposure estimations in high and frequent consumers varied from a minimum of 1.3 ng/kg bw per day in the adults group to a maximum of 11.8 ng/kg bw per day in the toddlers group.
High and frequent fish consumers were also present among pregnant women. The dietary exposure to MG/LMG for high and frequent fish consumers in this population group was calculated based on food consumption data originating from one survey. The 95th percentile dietary exposure estimate (4.1 ng/kg bw per day for pregnant women) was similar to that for the adults group.
| Age class | Number of surveys | 95th percentile hypothetical chronic dietary exposure in fish consumers | ||
|---|---|---|---|---|
| Minimum | Median | Maximum | ||
| Infants | 3 | 2.8 | 4.3 | 6.6 |
| Toddlers | 5 | 2.6 | 6.1 | 11.8 |
| Other children | 15 | 3.1 | 6.6 | 9.7 |
| Adolescents | 14 | 1.6 | 4.3 | 8.9 |
| Adults | 16 | 1.3 | 3.8 | 5.2 |
| Elderly | 10 | 1.5 | 3.6 | 5.2 |
| Very elderly | 6 | 2.3 | 3.5 | 4.4 |
- bw: body weight.
- a The minimum, median and maximum of the 95th percentile exposure values across dietary surveys (with more than 60 consumers) in European countries are shown.
- b This scenario contains all type of fish, fish products and crustaceans (excluding aquatic molluscs) that are contaminated with malachite green/leucomalachite green at a concentration equal to the reference point for action of 2 μg/kg.
The 95th percentile of the dietary exposure to MG/LMG is higher in the toddlers group for the high and frequent fish consumers, although it is not very different from that in the other children group. However, this was the reverse of that for the dietary exposure for all population, consumers and non-consumers. This is explained by the different percentage of ‘fish consumers-only’ between these two age groups. The toddlers ‘fish consumers-only’ account for 41% of the toddlers’ population, whereas the other children ‘fish consumers-only’ account for 32% of the other children population.
3.2.4 Non-dietary exposure
Additional exposure may occur from the permitted use of MG as an industrial dye for a wide range of manufactured goods. MG is commonly used in the manufacture of silk, jute, wool, cotton, leather, paper and acrylic products (Hidayah et al., 2013). Other uses of MG relate to its use as a biological stain for microscopic analysis, such as for bacterial cells, and LMG may be used in forensic science to identify the presence of blood (haemoglobin catalyses a reaction between colourless LMG and hydrogen peroxide to produce coloured MG). Another forensic use for MG is as a powder on money, leading to a green stain on the hands of a person handling the money.
Although the use of MG/LMG as a biological stain or in forensics is unlikely to result in substantial exposure of the general population, its use to dye manufactured products could result in exposure, particularly for certain groups, such as children, who might be inclined to sucking these products. In addition, there may be release of the dye into water sources and subsequent exposure through water and food. However, in the absence of data relating to the exposure of persons to MG from these various sources, an assessment of human non-dietary exposure is not possible.
3.3 Hazard identification and characterisation
3.3.1 Toxicokinetics
Despite its extensive use in the dyeing industry and in aquaculture, with the known presence of residues in aquatic organisms, there is limited knowledge on the kinetics of MG in mammalian and piscine species. Most of the available information is related to the biotransformation of the dye and the form(s) under which it may accumulate in edible tissues of fish and crustaceans (FAO/WHO, 2009).
In aqueous solutions, MG is in equilibrium with a colourless hydrated derivative referred to as MG carbinol or pseudobase (Figure 4).
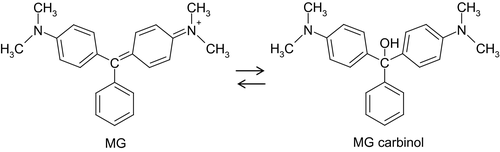
The rate of carbinol formation is negligible at pH < 2, slow at pH between 2 and 8, and very fast at pH 10 or higher, and may also increase with an increase in temperature (El Hajj Hassan et al., 2011). According to Albert (Albert, 1979 as cited in Srivastava et al., 2004), the carbinol derivative displays a higher liposolubility than MG and it is very likely that the dye is absorbed as MG carbinol by aquatic organisms.
Very limited information is available concerning MG absorption and disposition in mammals. Upon a single exposure of rats to radiolabelled MG, most of the radioactivity is found in the excreta (Law, 1994); in another study, a significant contribution from biliary excretion was reported in rats treated with unlabelled MG (Debnam et al., 1993).
In fish, MG is rapidly taken up mainly via the gills and both the absorption rate and peak blood concentrations are dependent on water temperature (Poe and Wilson, 1983; Alderman and Clifton-Hadley, 1993).
The main biotransformation reactions undergone by MG are reductive and oxidative in nature. Reduction of MG yields the colourless derivative LMG (Figure 1, see Section 1.3.3). This reaction may be accomplished by the intestinal microflora (Singh et al., 1994; Henderson et al., 1997); the role played by the gills in MG absorption and the extent of conversion of MG to LMG (see below) indicate that MG reduction to LMG could also occur in tissues, at least in piscine species. In treated fish, MG is rapidly cleared from the body while LMG is the predominant form in edible tissues where it may accumulate and persist for a long time due to a very slow excretion rate (Plakas et al., 1996; Alborali et al., 1997; Jiang et al., 2009).
No information could be retrieved concerning the nature of enzyme(s) performing the reductive biotransformation of MG to LMG in both laboratory and target species. An in vitro study was designed with the filamentous fungus Cunninghamella elegans, which has been used in several instances as a model for mammalian metabolism (Cerniglia, 1997). Based on the effects of nicotinamide adenine dinucleotide phosphate (NADPH) omission or the addition of metyrapone, a cytochrome P450 (CYP) inhibitor, it appears that CYP is involved in the generation of LMG (Cha et al., 2001).
In vivo studies carried out in mammalian species (Culp et al., 1999) have demonstrated that the oxidative MG pathway mainly entails the sequential N-demethylation of the dye to yield mono-, di-, tri, and tetra-desmethyl derivatives (Figure 5); the generation of MG N-oxides could also be demonstrated in liver (see below). The same metabolic pathway (i.e. a sequential N-demethylation) was also found to occur for LMG in either MG- or LMG-treated rats (Culp et al., 1999). The detection of both MG-N oxide and LMG N-demethylated metabolites in fillets from MG-treated catfish strongly supports the occurrence of a similar biotransformation pathway in fish (Doerge et al., 1998).
No papers could be found in the open literature on the identity of the enzyme(s) carrying out the oxidative metabolism of MG and LMG in vertebrates. The cited in vitro study, utilising cultures of C. elegans (Cha et al., 2001), provided indication of CYP involvement in the production of N-demethylated derivatives. The same conclusions were reached in a PhD dissertation (Giuliano Albo, 2003) in which the oxidative metabolism of the dye was investigated in rat and trout liver microsomes.
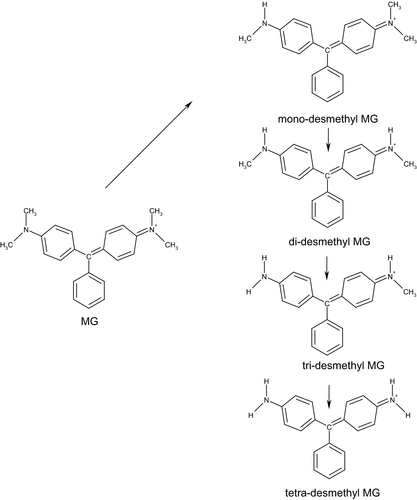
The capacity of extrahepatic enzymes to perform the N-demethylation of LMG was assessed in vitro; it could be demonstrated that, in the presence of tyrosine, iodide and H2O2, thyroid peroxidase (TPO) was effective in the biotransformation of LMG, generating desmethyl LMG, di-desmethyl LMG, tri-desmethyl LMG, MG itself, and MG N-oxide (Doerge et al., 1998). This pathway has been implied in the LMG-mediated competitive TPO inhibition, which could explain the negative effects on thyroid function (decreased thyroxine (T4) and increased thyroid-stimulating hormone (TSH)) observed in LMG-treated rats (Culp et al., 1999).
The primary and secondary amines resulting from the sequential N-demethylation of MG and LMG closely resemble carcinogenic amines such as gentian violet or pararosaniline (Doerge et al., 1998). The previously mentioned isolation of MG N-oxides from liver extracts of MG-treated rats supports the hypothesis that, similar to many carcinogenic amines, the N-demethylated metabolites may enter an oxidative bioactivation pathway (N-hydroxylation followed by the formation of N-oxides; Figure 6) generating reactive intermediates, which have been associated with the formation of liver- and thyroid DNA adducts as detected by 32P-postlabelling in treated animals (Culp et al., 1999).
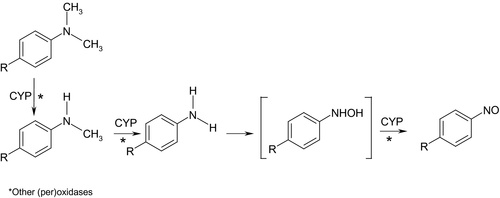
Little is known about the role of phase II enzymes in MG/LMG biotransformations. In MG-treated rats, evidence has been provided concerning the formation and the biliary excretion of an MG-glutathione (GSH) adduct (Debnam et al., 1993); however, a direct involvement of GSH-S-transferases (GSTs) has not been demonstrated, the enzyme(s) being instead strongly inhibited by the dye (see below). The spontaneous in vitro formation of MG adducts with GSH and other (protein) thiols (Figure 7) has been thoroughly characterised by indirect spectrophotometric methods (Reuben and Bruice, 1976; Debnam et al., 1993; Eldem and Özer, 2004), although no such studies have been performed with MS techniques that would allow identification of the GSH-conjugates.
A number of in vitro studies indicate that, in tissue preparations, MG is able to inhibit a number of xenobiotic metabolising enzymes including several rat liver CYP-dependent monooxygenases and the NADPH CYP-reductase (Beyhl, 1981) as well as hepatic rat (Debnam et al., 1993) or human (Glanville and Clark, 1997) GSTs. The relevance of the results of such studies under in vivo conditions remains to be established.
3.3.1.1 Laboratory animals
In vivo studies
Rats of both sexes (n = 3) were administered a single dose of 14C-MG (2 mg/kg bw) by gavage, and faeces and urine were collected daily. Animals were sacrificed after 7 days and samples of kidney, liver, muscle and blood were taken. At that time, 14C equivalents were in the sub μg/kg range and in the order liver > kidney > skin > muscle.23 Over the whole study period, most of the radioactivity (mean ± standard deviation (SD) = 96 ± 6%) was recovered from the excreta; at each time point, faecal radioactivity was largely prevailing over urinary radioactivity, accounting for more than 80% of the cumulative 14C excretion. Due to low levels of blood and tissue radioactivity, the nature of residues was not investigated further (Law, 1994). Biliary excretion was studied in rats with a cannulated bile duct (number not specified) treated with MG (2–7 mg/kg bw) by the intravenous route; it was found that MG is rapidly excreted via the bile, very likely as a GSH adduct, reaching a peak 20 min after dosing (Debnam et al., 1993).
The nature and the amount of MG/LMG metabolites were investigated in rat and mice liver extracts after a short-term feeding study with either MG or LMG (Culp et al., 1999). Male Fischer 344 rats and female B6C3F1 mice (6–7 week old, n = 8 per dose group) were fed 0, 100, or 600 mg MG/kg diet or 0, 96, or 580 mg LMG/kg diet. After 28 days of feeding, animals were sacrificed and liver extracts were subjected to chemical analysis by HPLC-UV and, for rats only, by HPLC in combination with atmospheric pressure chemical ionisation–mass spectrometry (APCI–MS). With APCI–MS analysis, liver extracts from MG fed rats showed the presence of mono-, di-, tri-, and tetra-desmethyl MG derivatives as well as MG N-oxide and a small but measurable amount of LMG. The quantitative analysis of liver extracts from treated rats and mice was performed by HPLC-UV. In MG-fed animals, the dye itself was the most abundant compound followed by mono- and di-desmethyl MG and LMG, and, in the case of rats, also mono- and di-desmethyl LMG. A dose-related increase in the concentration of MG and its metabolites was observed. A more complex picture was observed in chromatograms from rats administered LMG, showing not only LMG and four N-demethylated derivatives, but also MG mono- and di-desmethyl derivatives, MG N-oxide and a little but measurable amount of MG itself.
In vitro interactions with xenobiotic metabolising enzymes
A study was designed to investigate the in vitro interactions of triphenylmethane dyes (including MG) with hepatic xenobiotic metabolising enzymes. The incubation of MG 0.1 mM with rat liver preparations resulted in the inhibition of different CYP-dependent monooxygenases to an extent ranging from about 90% (aminopyrine N-demethylase) to about 50% (anisic ester O-demethylase), while NADPH CYP-reductase was also inhibited by 30%. Most of the tested triphenylmethane dyes inhibited the different monooxygenases to a similar extent. It was concluded that the observed dye-mediated inhibition could be due not only to a direct interaction with the haemoprotein, but also to the redox properties of many tested compounds – including MG – hampering the electron transfer to the CYP-complex by NADPH CYP-reductase (Beyhl, 1981).
The interactions between MG and GSTs were investigated in rat liver preparations. The dye was found to inhibit the tested GST classes (1-1 and 1-2) by a partial non-competitive mechanism, with a inhibition constant (Ki) in the micromolar range; the MG–GSH adduct was believed to contribute significantly to the observed inhibition (Debnam et al., 1993).
3.3.1.2 Humans
No data on the toxicokinetics of MG in humans could be found in the open literature. The only reference to human data is the in vitro capacity of human faecal microflora to almost completely reduce MG to LMG (Henderson et al., 1997).
The three main human GST classes (α, μ, π)25 were expressed in Escherichia coli and tested for their sensitivity to inhibition by MG and other basic triphenylmethane dyes. All three GST classes were inhibited by MG in a non-competitive manner displaying Ki values in the order of 0.3 μM (π), 10 μM (α) or 70 μM (μ). Despite GSH–MG adduct formation being demonstrated, the nature of the inhibitor (adduct or free dye) was not established (Glanville and Clark, 1997).
3.3.1.3 Fish and crustaceans
Fish
Uptake, accumulation and depuration of MG has been studied in herbivorous, omnivorous and carnivorous freshwater fish (Parabramis pekinensis, Carassius auratus and Ophiocephalus argus, respectively) following acute aquatic exposure (6 mg/L for 20 min) (Jiang et al., 2009). MG and LMG concentrations were measured at six time points (0, 2, 4, 24, 96 and 240 h) in blood, muscle, liver, kidney, gill and skin from all three fish species (and in abdominal fat of P. pekinensis, which was present in the other two species at insufficient quantities for dissection). The highest concentrations of MG were in gill tissue of fish sampled immediately after exposure (P. pekinensis, 5.1 mg/kg; C. auratus, 5.6 mg/kg and O. argus, 7.2 mg/kg). Most MG was rapidly converted to LMG in the fish and depuration of LMG was slow in fat tissue, skin and gonads of the fish. The highest mean MG level in fat in P. pekinensis was 2.4 mg/kg at 24 h, whereas LMG concentrations increased with time, achieving a maximum concentration of 8.5 mg/kg at 240 h. Distribution of LMG in fish was dependent on the fat content in the tissue, but not related to the different feeding habits of the fish species.
The effects of salinity and fish size on elimination of MG was investigated in tilapia (Oreochromis spp.) by Yang et al. (2010). Small tilapia (45 ± 10 g; mean ± SD) were exposed to 0.5 mg or 2 mg MG/L for 1 h in fresh water, or 2 mg MG/L in brackish water (15‰ salinity). The fourth treatment involved large tilapia (150 ± 20 g), which were exposed to 2 mg MG/L for 1 h in fresh water. Both MG and LMG were rapidly eliminated from tilapia muscle tissue with elimination of MG being more rapid than that of LMG. The elimination of both MG and LMG was faster from large fish than from small fish. Salinity of the pond water had no apparent effect on the elimination rate of MG or LMG from small tilapia. Higher MG and LMG concentrations were present in fish from the high exposure group (2 mg/L) than in the low-dose group (0.5 mg/L), the elimination rates being comparable at both concentrations such that the residues were detected for a longer time in fish exposed to 2 mg/L.
MG residues in muscle, eggs, and fry of Atlantic salmon (Salmo salar) and chinook salmon (Oncorhynchus tshawytscha) were determined colorimetrically after repeated exposure (10–47 times) to 1 mg/L for 1 h at fish hatcheries (Allen, 1990). Residues in eggs from treated females were 0.1–4.2 mg/kg in Atlantic salmon and 0.1–1.0 mg/kg in chinook salmon; with little relation between the residue concentrations in the eggs and the time elapsed since the last treatment. Residues of MG in fry from treated chinook salmon ranged from 0.14 to 1.16 mg/kg.
Kasuga et al. (1992) examined the elimination of MG from rainbow trout (Oncorhynchus mykiss) following exposure to 1 mg/L for 1 h. Highest concentrations of MG were found in kidney (approximately 3.4 mg/kg after 2 h) and liver (approximately 2.4 mg/kg immediately following treatment) and lower concentrations in serum (approximately 0.8 mg/kg immediately following treatment) and muscle (approximately 0.5 mg/kg after 4 h). MG in serum, liver and muscle gradually decreased over time and levels were below the LOD (20 μg/kg) after 21 days. The depletion from kidney was slow, and at the end of the study (42 days) the MG level was 0.22 mg/kg.
Máchová et al. (1996) exposed rainbow trout to 0.2 mg MG/L for 6 days. Immediately after treatment, the sum of MG and LMG concentrations in muscle, liver and skin were 0.71, 0.83 and 0.65 mg/kg, respectively. Eight weeks after exposure, MG was below the LOD (1 μg/kg) in muscle and liver, whereas it was measurable in skin (0.011 mg/kg). The sum of MG and LMG was still detectable in fish skin 10 months after exposure whereas after 12 months no residues were detected.
Rainbow trout (60–80 g) were exposed to MG (1 mg/L) for 1 h and then put into clean water. The same treatment was repeated for a total of 7 days. Total tissue MG residues were determined after LMG oxidation with PbO2 with an HPLC method (LOD: 1 μg/kg). MG concentrations in fillets peaked at about 30 days after cessation of treatment (nearly 6 mg/kg) were around 0.2 mg/kg at 90 days, and then slowly declined reaching the LOD value (1 μg/kg) at 283 days post-treatment (Alborali et al., 1997). Alderman and Clifton-Hadley (1993) examined the uptake, distribution and elimination of MG in rainbow trout following exposure to 1.6 mg/L for 40 min, and water temperature maintained at either 8°C or 16°C. All three processes were temperature dependent, with rates and levels increasing with higher water temperature. Peak MG concentrations were found in serum (13 mg/L), liver (9 mg/kg) and kidney (8 mg/kg) immediately after exposure, at 8°C, and in kidney (34 mg/kg), liver (16.5 mg/kg) and serum (13.5 mg/L) at 16°C. Peak MG levels in muscle were found after 90–120 h, and were 7.8 mg/kg at 8°C and 10.8 mg/kg at 16°C. Elimination half-lives of MG from tissue were 30, 13 and 4 days at 8°C, and 5.8, 5.7 and 2.9 days at 16°C for kidney, liver and muscle, respectively. Residues in kidney above 1 mg/kg were detected after 24 days. Law (1994) exposed trout at 10°C to 2 mg 14C-labelled MG/LMG for 1 h and sampled fish at intervals until 505 h post treatment. The highest concentrations of residues were found in liver and kidney; residues were also found in skin. Elimination half-lives were 28 h for MG and 197 h for LMG.
Bajc et al. (2011) examined the uptake, accumulation and depuration of MG in muscle and skin of rainbow trout and carp. Rainbow trout was exposed to 1 mg MG/L for either 1 or 3 h, or 1.5 mg/L for 1 h. Increasing the dose of MG did not significantly affect the residue levels in rainbow trout (0.45 and 0.53 mg MG/kg in the 1 and 1.5 mg/L groups, respectively); however, exposure duration had a major influence. Rainbow trout exposed three times longer contained 4.5 times more MG (2.1 mg/kg) and two times more LMG (6.0 mg/kg) 1 day after exposure compared with the trout in the 1-h bath treatment (0.45 mg MG/kg and 2.8 mg LMG/kg). The LMG concentration in trout exposed to 1 mg MG/L for 3 h continued to increase after removal from the treatment, reaching up to 9.7 mg LMG/kg after 4 days. The level of MG in rainbow trout decreased rapidly and, 1 day after treatment, about 74% of total residues were in the leuco-form. Levels of MG in fish fell below the CCβ (0.6 μg/kg) after 58 days in the group exposed to 1 mg/L for 1 h, and below the CCβ after 142 days in the group exposed to the same concentration for 3 h. Carp were exposed to higher concentrations of MG than rainbow trout; 2 mg/L for 1 or 3 h, or 4 mg/L for 1 h. Nevertheless, they accumulated less MG than rainbow trout and the elimination of LMG was slower, which may reflect the lower water temperature used for carp.
Mitrowska et al. (2008b) investigated the tissue distribution and persistence of MG and LMG in carp after exposure to 2 mg MG/L for 3 h. MG was rapidly and extensively metabolised to LMG, which was slowly eliminated from the tissues. Higher concentrations of MG and LMG were found in the gills, liver and kidney than in the spleen and muscle. MG was more persistent in kidney, liver and spleen (up to 112 days, LOQ: 0.5 μg/kg) than in the gills and muscle tissue (up to 56 days). LMG concentration declined more slowly than MG in all tissues, and was still detectable in kidney and muscle 252 days after treatment.
The residue pattern of MG and its metabolites was studied in fillets from mature catfish using an LC/MS method with LODs of 0.02 and 0.5 μg/kg for MG and LMG, respectively (Doerge et al., 1998). Fish were exposed to 1 mg/L MG oxalate for 1 h, then transferred to a tank containing clean water and killed 24 h after dosing. The concentrations of LMG in fillets were almost twice those of MG; further metabolites were also detected including MG N-oxide and the mono-, di-, and tri-desmethyl LMG derivatives (not quantified). In the same study, the presence of the same analytes was also investigated in trout fillets purchased from retail outlets in the UK in 1994–1995. The concentrations of MG ranged from 0.0004 to 0.0034 mg/kg while those of LMG ranged from 0.009 to 0.096 mg/kg; signals consistent with the presence of MG N-oxide and both mono- and di-desmethyl MG were also observed, and in one fillet sample they were estimated to be 0.035, 0.018 and 0.006 mg/kg, respectively. The authors concluded that the occurrence of metabolites bearing the structure of primary arylamines in fish fillets might represent a potential concern for the consumer.
Plakas et al. (1996) examined the distribution of radiolabelled MG in channel catfish (Ictalurus punctatus) for 2 weeks after waterborne exposure to 0.8 mg/L for 1 h and also the effects of pH 6, 7 or 8 on MG and LMG accumulation. Mean water quality variables were 21°C, dissolved oxygen was 8 mg/L, and the pH was 7.1 in the control group. The peak concentrations of radioactive residue in bile, plasma and tissue are shown in Table 3.
| Tissue | Concentration (mg equivalents MG/kg or L) | Time after cessation of exposure (h) |
|---|---|---|
| Bile | 101 ± 41.2 | 24 |
| Liver | 33.4 ± 7.45 | 0 |
| Fat | 30.3 ± 6.39 | 168 |
| Head kidney | 24.6 ± 4.86 | 0 |
| Trunk kidney | 22.0 ± 5.38 | 0 |
| Spleen | 10.9 ± 2.49 | 0 |
| Plasma | 6.36 ± 1.51 | 0 |
| Skin | 3.99 ± 0.68 | 0 |
| Muscle | 3.18 ± 0.72 | 0 |
- MG: malachite green.
- Mean ± standard deviation for five animals per time point.
MG in plasma had an elimination half-life of 4.7 h, whereas in muscle the elimination half-life was approximately 67 h. MG was rapidly and extensively metabolised to LMG, which was slowly eliminated from the tissues. The rate of MG accumulation was related to pH of the exposure water. The sum of MG and LMG concentrations was eight times higher in plasma (approximately 16 mg/L vs 2 mg/L) and five times higher in muscle (approximately 3 mg/kg vs 0.6 mg/kg) after exposure at pH 8 compared to MG levels in fish treated at pH 6.
The accumulation, distribution and excretion of MG and LMG were examined in channel catfish blood and tissue (muscle, liver, skin and kidney) following exposure to 7 mg MG/L for 5 min (Liu et al., 2013). Experimental conditions were monitored daily, the average water temperature was 26.4°C and the pH was 7.5–8.0. Peak concentrations of MG and LMG in blood were 0.118 and 0.203 mg/L after 4 and 6 h, respectively. The elimination half-lives for MG and LMG in blood were 113 and 106 h, respectively. The peak concentration of MG in skin was 2.03 mg/kg after 4 h, in muscle 0.49 mg/kg after 1 h, in kidney 0.27 mg/kg after 0.5 h and in liver 0.14 mg/kg after 4 h. The half-lives of MG in muscle, skin, liver and kidney were 3.2, 4.1, 4.8 and 14.4 days, respectively. MG was not detectable in liver after 30 days, in muscle after 45 days, and in skin or kidney after 60 days. The peak concentration of LMG in liver of channel catfish was 5.58 mg/kg after 0.5 h, in skin and muscle 1.21 and 0.87 mg/kg, respectively, after 8 h, and in kidney 0.36 mg/kg after 48 h. The half-lives of LMG in muscle, skin, liver and kidney were 7.2, 9.6, 4.1 and 14.4 days, respectively. LMG was not detectable in liver after 60 days, or in the other tissues at the end of the experiment, namely 90 days.
Yang et al. (2013a) examined the elimination of MG and LMG from Channel catfish following the administration of 1 mg/L to the water (analysed peak water concentration 46 μg MG/L after 24 h). Dorsal muscle and skin were analysed for MG and LMG after 1, 3, 5, 10, 15, 30, 45, 60, 90, 120, 180, 210, 240, 270, 300, 330 and 360 days following exposure. One day after exposure, the peak concentrations of MG in muscle and skin tissue were 42.77 ± 5.26 and 6.36 ± 0.11 μg/kg, respectively, and elimination half-lives were 57.8 days and 31.5 days, respectively. The peak concentrations of LMG in muscle (502.3 ± 20.43 μg/kg) occurred 3 days after exposure whereas the highest concentration in skin (125.3 ± 12.8 μg LMG/kg) occurred after 1 day. Elimination half-lives for LMG were 33.0 and 38.5 days for muscle and skin, respectively.
The elimination of MG and LMG was studied for 100 days in glass eels (Anguilla anguilla) following exposure to 0.1 mg MG/L for 24 h (Bergwerff et al., 2004). The highest mean MG concentration in eel, 4.35 ± 0.59 mg/kg (mean ± SD) was found 6 h after treatment commenced. The LMG levels in eel were relatively high (> 0.1 mg/kg) from about 2 h after treatment, with a maximum LMG concentration of 0.83 ± 0.23 mg/kg occurring after 3 days. LMG was eliminated slowly from eel and residues remained 100 days after exposure (0.015 mg/kg).
Crustaceans
The accumulation and elimination of MG was studied in Pacific white shrimp (Litopenaeus vannamei) following exposure to 0.15 mg/L for 2 h (Yang et al., 2013a). Experimental conditions were: water temperature: 28–33°C, pH: 7.8–7.9, salinity: 28‰, dissolved oxygen: 6.83–7.11 mg/L. Peak concentrations of MG and LMG were found immediately after exposure (0.273 and 0.519 mg/kg, respectively). MG was eliminated rapidly from the shrimp, and was below the LOD (0.5 μg/kg) after 3 days, whereas LMG elimination was slower, being below the LOD after 9 days. Similarly, Chen et al. (2013) found peak levels of MG and LMG in Pacific white shrimp muscle and head immediately after exposure to 0.2 mg/L for 2 h (0.02 mg MG/kg and 0.079 mg LMG/kg, and 0.185 mg MG/kg and 0.392 mg LMG/kg in muscle and head, respectively). Experimental conditions were: water temperature: 25–30°C, pH: 7.7–7.8, salinity: 28‰, dissolved oxygen: 6.7–7.2 mg/L. Both MG and LMG elimination were more rapid from head than muscle tissue, and LMG was eliminated from both tissues more slowly than MG (MG was below the LOD (0.5 μg/kg) in shrimp head after 3 days and in muscle after 4 days, whereas LMG was below the LOD in shrimp head after 5 days and in muscle after 7 days. The difference in results from the two studies may be accounted for by the different life stage used in the two experiments; Yang et al. (2013b) used shrimp seed of approximately 0.9 cm, whereas Chen et al. (2013) conducted their study on prawns of approximately 4–5 cm and weight of 1.33–2.41 g. Consequently, the whole animals were analysed for MG and LMG in the former trial, whereas the latter examined concentrations in head and muscle tissue separately.
Exposure to LMG through feed
Seel-audom et al. (2013) examined the accumulation and effects of diet-borne LMG in Nile Tilapia (O. niloticus) fed experimental diets containing nominal concentrations of 0, 0.1, 0.5 or 2.5 mg LMG/kg (measured values 0.104, 0.510, and 2.2 mg/kg) for 28 days. The concentrations of LMG in liver and muscle were 0, 0.0119, 0.0483, 0.231 mg/kg in liver and 0, 0.0068, 0.0262, and 0.0917 mg/kg in muscle, respectively.
3.3.1.4 Accumulation in edible tissue
Maximum levels of MG in edible tissue of fish, i.e. in muscle, occur immediately after exposure whereas peak levels of LMG tend to occur later (hours to days) depending on the fish species, the MG exposure concentration and duration, and environmental conditions such as temperature and pH. Limited studies on crustaceans (shrimp) indicate that levels of both MG and LMG fell below the LOD (0.5 μg/kg) after some days following treatment.
3.3.1.5 Conclusions
There is limited knowledge on the kinetics of MG and its metabolites in mammalian and piscine species, the published studies being mostly focused on occurrence of the parent compound and its derivatives in tissues. The main metabolite is LMG, the reduced form of MG, which is slowly excreted and may be stored in fish edible tissues for a long time after exposure. An oxidative pathway, likely mediated by CYPs and other (per)oxidases, has been demonstrated entailing the sequential N-demethylation of both compounds. This results in the formation of N-demethylated metabolites (structurally similar to carcinogenic amines), which have been found to occur in fish fillets. Apart from the likely spontaneous generation of a MG–GSH adduct, which is reported to undergo biliary excretion in rats, no information could be found on the involvement of other conjugation reactions in MG/LMG biotransformation.
3.3.2 Toxicity in experimental animals
3.3.2.1 Acute toxicity
For female Sprague–Dawley rats an oral median lethal dose (LD50) for MG-oxalate (purity not given) of 520 mg/kg bw (95% confidence limits 433–624 mg/kg bw) was reported by Meyer and Jorgenson (1983). Observed effects included depression, prostration, emaciation, coma and death. The oral LD50 for MG-oxalate (purity ≥ 90%) in Wistar rats was 275 mg/kg bw. No sex differences were observed. Major acute effects were hyperaemia and atonia of the intestinal walls, often in association with dilatation of the gastrointestinal tract (Clemmensen et al., 1984).
3.3.2.2 Repeated dose toxicity
Groups of male and female B6C3F1 mice (8/sex per dose group) received a diet containing 0, 25, 100, 300, 600 or 1,200 mg MG/kg diet (corresponding to 0, 4, 18, 50, 100 or 220 mg/kg bw per day for males and 0, 5, 20, 65, 120 or 250 mg/kg bw per day for females) for 28 days. Purity of MG was ≥ 94%. Female mice at the highest dose showed a decrease in final body weight (to about 92% of controls). Feed consumption was not affected. Significant but small decreases (< 7%) in erythrocyte count, haemoglobin and haematocrit were found in females at 120 and 250 mg/kg bw per day and in males in the highest dose group. Reticulocytes were significantly increased in females at doses of 65 mg/kg bw per day and higher, but in males at the highest dose only. No significant histopathological changes were observed (Culp et al., 1999; NTP, 2004).
MG-oxalate (purity ≥ 90%) was administered to groups of male and female Wistar rats (8/sex per dose group) in the diet at concentrations of 0, 10, 100 or 1,000 mg MG-oxalate/kg diet for 28 days. Age of the animals was not reported, but body weights at the start of the experiment ranged from 170 to 250 g. These concentrations correspond to doses of 0, 1.2, 12 and 120 mg MG-oxalate/kg bw per day (equivalent to 0, 0.9, 9.4 and 94.5 mg MG/kg bw per day) when applying a default factor of 0.12 for a subacute study (EFSA Scientific Committee, 2012). No clinical signs were seen at 0.9 or 9.4 mg MG/kg bw per day, but hyperactivity and a significant reduction in body weight gain and in food consumption (none of these parameters quantified) were observed at 94.5 mg MG/kg bw per day in both sexes. High-dose females showed small haematological changes: an increase in lymphocytes, a decrease in neutrophils and a decrease in haematocrit. In high-dose males, a significant increase in plasma urea was noted (Clemmensen et al., 1984).
Male and female Fischer 344 rats (8/sex per dose group) were administered a diet containing 0, 25, 100, 300, 600 or 1,200 mg MG/kg diet (purity ≥ 94%) (corresponding to 0, 3, 12, 40, 70 or 175 mg/kg bw per day for males and 0, 3, 12, 40, 75 or 190 mg/kg bw per day for females) for 28 days. A decrease in mean body weight (to about 80% of the control for females and to 87% of the control for males) was found at the highest dose. Final body weight was significantly reduced for females at 75 and 190 mg/kg bw per day for females and at 175 mg/kg bw per day for males. Feed consumption was generally similar to that of the controls. Absolute and relative liver weights were significantly increased in females from 40 mg/kg bw per day onwards and in males in the two highest dose groups. Histopathological examination revealed an increased incidence of hepatocyte cytoplasmic vacuolisation in male rats in the two highest dose groups, 1/8 and 4/8, respectively, compared to 0/8 in the control and other groups, and of 7/8 in the highest dose females, compared to 0/8 in the control and other groups. No further details on this effect were provided. In both sexes, there was a significant increasing trend in the activity of gamma-glutamyl transferase (GGT), being 4.2-fold greater in high-dose females than in the controls. Red blood-cell parameters (erythrocyte count, haemoglobin and haematocrit) showed a small (< 5%), but significant, decrease in females of the highest dose group. Male rats showed a small (< 3%), but significant, decrease in mean erythrocyte haemoglobin at 40 mg/kg bw and higher, however without a consistent dose–response relationship. No effect on reticulocytes was observed in male and female rats (Culp et al., 1999; NTP, 2004).
Male and female Fischer 344 rats (8/sex per dose group) were fed a diet containing 0 or 1,200 mg MG/kg diet (equivalent to approximately 0 or 200 mg/kg bw per day) for 4 or 21 days (purity ≥ 94%). Triiodothyronine (T3) levels were significantly higher in treated females at 21 days than in controls, and T4 levels were significantly lower in females at 4 and 21 days. There were no significant changes in T3 and T4 levels for males and no significant changes in TSH for either sex (Culp et al., 1999; NTP, 2004).
Groups of female B6C3F1 mice (8/dose group) were fed a diet containing LMG (purity ≥ 99%) at concentrations of 0, 290, 580 or 1,160 mg/kg diet for 28 days. These concentrations correspond to doses of about 0, 60, 110 or 220 mg/kg bw per day. Final body weight and absolute and relative kidney weight were significantly decreased in the two highest dose groups. Feed consumption was lower in all dose groups, on average about 80–90% of the controls, but without a dose–response relationship. All mice at the high dose had scattered dead or degenerated cells in the transitional epithelium of the urinary bladder. Many of the cells lacked nuclei, and when visible, the nuclei were condensed or fragmented, suggesting apoptosis (Culp et al., 1999; NTP, 2004).
LMG (purity ≥ 99%) was administered to male Fischer 344 rats (8/dose group) in the diet at concentrations of 0, 290, 580 or 1,160 mg/kg diet (corresponding to 0, 30, 60 or 115 mg/kg bw per day) for 28 days. Final body weight was significantly decreased in the high-dose group. Feed consumption was on average about 10% lower in the two highest dose groups, compared to the controls. Small (< 6%) but significant decreases of haemoglobin, haematocrit and the erythrocyte count were observed in the high-dose group. A significant increase in the GGT activity and phosphorous level was observed in the high-dose group. Absolute liver weight increased with dose, but the increase was significant at the high-dose only. A significant dose-related increase in relative liver weight was found in all groups. A dose-related increase in hepatocyte cytoplasmic vacuolisation was observed (0/8, 2/8, 5/8, 7/8 for control, low- , mid-, and high-dose groups, respectively), being significant in the mid- and high-dose group. This effect was not further characterised. Upon histological examination, also apoptotic follicular epithelial cells in the thyroid gland were observed at the two highest doses (2/8 in the 60 and 2/8 in the 115 mg/kg bw per day groups), and there was evidence of follicular epithelium regeneration (Culp et al., 1999; NTP, 2004).
Male Fischer 344 rats (8/dose group) were fed a diet containing 0 or 1,160 mg LMG/kg diet (equivalent to approximately 0 or 200 mg/kg bw per day) for 4 or 21 days (purity of LMG ≥ 99%). There was a significant decrease in T4 and a significant increase in TSH levels at days 4 and 21 compared to the controls. T3 levels were not affected (Culp et al., 1999; NTP, 2004).
Conclusion
Information on the repeated dose toxicity of MG and LMG in mice and rats was confined to 28-day toxicity tests only. MG caused haematological effects in mice and rats and an increase in absolute liver weight in male and female rats. The overall NOAEL, based on haematological effects in female rats, was 9.4 mg/kg bw per day. LMG decreased body weight and induced histopathological changes in the bladder of mice at the highest dose (220 mg/kg bw per day). In male rats, absolute liver weight was increased at the highest dose, but the incidence of hepatocyte cytoplasmic vacuolisation was increased at all doses tested. Consequently, no NOAEL can be identified for LMG.
3.3.2.3 Immunotoxicity
No immunotoxicity studies with oral administration were identified.
3.3.2.4 Developmental and reproductive toxicity
MG oxalate (purity not given) was administered by gavage to groups of 20 pregnant New Zealand white rabbits at doses of 0, 5, 10 or 20 mg MG oxalate/kg bw per day from day 6 through 18 of gestation (Meyer and Jorgenson, 1983). Intubation errors led to loss of four animals in both the low- and high-dose groups. Body weight loss occurred in dams of the two highest dose groups. The body weights of the progeny of the treated rabbits were less than those of the controls (37.1, 34.2, 35.8 and 35.2 g for control, low-, mid- and high dose, respectively), but differences were only significant at the low- and high dose. Fetal toxicity was shown at all three dose levels. There were significant increases in preimplantation losses, but actual data on this parameter were not reported by the authors. Based on the reported data for total number of corpora lutea and total number of implantation sites, the CONTAM Panel calculated that the preimplantation loss was 8%, 19%, 20% and 17% for control, low-, mid- and high dose, respectively. At all three dose levels, the average number of resorptions per dose was significantly increased (0.8, 2.1, 2.3 and 3.8 for control, low-, mid- and high dose, respectively) and the average number of live fetuses per dose was significantly decreased (7.3, 4.9, 5.2, 5.2 for control, low-, mid- and high dose, respectively). According to the authors, early resorptions accounted for most of the dead implants, but detailed information was not provided. An increased incidence in skeletal malformations (incomplete ossification of caudal vertebrae and malformed skulls) was observed in all treated groups (20%, 36%, 38% and 38% for control, low-, mid- and high dose, respectively). In treated animals, enlargement of the liver and heart and haemorrhaging of the bladder was also observed, but without a dose–response relationship. The CONTAM Panel considered this study to be of limited value, because of the losses sustained during gavage of the animals, the inadequate reporting and the lack of a consistent dose–response relationship for the observed fetal toxicity and skeletal and visceral malformations.
LMG (Purity 99.0%) was administered orally (by gavage) to groups of 24 female Sprague–Dawley rats of 11–12 weeks of age, mated with males of the same strain (Wan et al., 2011). Administration of LMG was once daily from day 6–15 of gestation, at doses of 0, 10, 80 or 160 mg/kg bw per day. At doses of 80 and 160 mg/kg bw per day, LMG induced maternal toxicity, which consisted of a severe reduction in maternal weight and feed consumption compared to the controls. The number of corpora lutea per dam, number of implants per dam, and preimplantation losses per dam were not affected by LMG, but post-implantation losses per dam and the number of dams with resorptions were significantly increased in the highest dose group. Fetal body weight and placental weight were significantly decreased at 160 mg/kg bw per day only. In the treated groups, the incidences of fetuses (and litters) with external abnormalities were not significantly different from those in the control group. LMG induced a dose-related increase in the incidence of fetuses (reported as 8.8%, 13.8%, 36.6% and 72.3% for the control, low-, mid- and high dose, respectively) and litters (reported as 34.8%, 58.3%, 73.7% and 100% for the control, low-, mid- and high dose, respectively) with skeletal abnormalities. The predominant skeletal abnormalities were bipartite ossification of the thoracic centrum (reported as 11%, 19%, 41% and 45% of the fetuses for the control, low-, mid- and high dose, respectively). These effects were significant at doses of 80 mg/kg bw per day and higher. The CONTAM Panel noted that the number of fetuses with skeletal abnormalities for each litter was not reported. In the high-dose group (160 mg/kg bw per day), LMG also induced a significant increase in the number of fetuses and litters (%) with visceral abnormalities, particularly hydronephrosis, compared with the controls. Based on the observed effects, a NOAEL of 10 mg/kg bw per day for maternal and fetal developmental toxicity in rats could be identified.
The developmental toxicity of MG has also been studied in a model with zebrafish (Danio rerio) embryos. Exposure of zebrafish embryos to MG concentrations of 0.125, 0.15 or 0.175 mg/L for 14 h significantly altered cardiovascular development causing growth retardation through blocking of the activation of the vascular endothelial growth factor receptor 2. In contrast, exposure up to 0.875 mg LMG/L for 14 h did not affect zebrafish embryo growth (Jang et al., 2009).
Minta and Wilk-Zasadna (2007) studied potential embryotoxic activity of MG and LMG in an in vitro model using micromass cultures of rat embryonic mesenchyme limb and midbrain cells. Embryonic mesenchyme limb and midbrain cells cultured at high density were exposed to 0.002, 0.005, 0.013, 0.032, 0.080, 0.200, and 0.500 μg/mL MG and 0.03, 0.12, 0.49, 1.95, 7.81, 31.25, and 125 μg/mL LMG. After 5 days of incubation, the viability and differentiation were determined. From the dose–response curves, the concentrations (μg/mL) that produced 50% inhibition of viability/proliferation (IC50-P) and 50% reduction of differentiation (IC50-D) were calculated. Embryotoxicity was classified according to the prediction model described by Genschow et al. (2002). MG and LMG induced dose-dependent inhibition of cell survival and differentiation in both cell cultures. MG was approximately 200–300 times more potent than LMG. The reduction of cell survival and differentiation were observed at similar concentrations. IC50-P values ranged from 0.02–0.08 and 5–17 μg/mL for MG and LMG, respectively. According to the prediction model, MG was classified as strongly embryotoxic in mesenchyme limb cells (IC50-D = 0.02 μg/mL) and midbrain cells (IC50-D = 0.05 μg/mL), whereas LMG was classified as weakly embryotoxic in mesenchyme limb cells (IC50-D = 15.6 μg/mL) and strongly embryotoxic in midbrain cells (IC50-D = 3.7 μg/mL).
Conclusion
Both MG and LMG induced developmental effects. MG caused fetal toxicity and skeletal malformations in rabbits at all doses tested (5–20 mg/kg bw per day). However, the CONTAM Panel noted that these effects did not show a consistent dose–response relationship, probably due to the small dose range of the study. For LMG, a NOAEL of 10 mg/kg bw per day for fetal toxicity in rats was identified, although there is no specific information on the individual litters. The CONTAM Panel noted, however, that these effects of LMG were observed at doses also causing maternal toxicity.
3.3.2.5 Neurotoxicity
No neurotoxicity studies with oral administration were identified.
3.3.2.6 Genotoxicity
The in vitro and in vivo genotoxicity studies for MG and LMG are summarised in Tables 4 and 5, respectively.
Bacterial reverse mutation assays (Ames test) of MG were performed with Salmonella Typhimurium strains TA97, TA98, TA100, TA102, TA104, TA1535 and TA1537. Because MG is highly toxic to bacteria, it was tested only at low doses, with a few exceptions. In one study, MG was mutagenic only in TA98 in the presence of S9 (Clemmensen et al., 1984). In later studies, the compound was tested at doses up to 10 μg/plate only, due to its antibacterial activity, and did not show any mutagenic activity (Fessard et al., 1999; NTP, 2004). LMG showed no mutagenic activity at doses up to 2,000 μg/plate (Fessard et al., 1999).
Assessment of the in vitro mutagenicity of MG or LMG in the Chinese hamster ovary (CHO) hypoxanthine–guanine phosphoribosyltransferase (HPRT) locus assay gave negative results, with and without S9 activation (Fessard et al., 1999). Due to severe cytotoxicity, evaluation of the mutagenicity of MG was restricted to very low concentrations (up to 0.05 μg/mL and 1 μg/mL without and with metabolic activation, respectively). LMG was less cytotoxic than MG and mutagenicity of LMG was evaluated at concentrations of 5–100 μg/mL.
In CHO cells, the capacity of MG and LMG to induce DNA damage was determined by the Comet assay (Fessard et al., 1999). When tested without metabolic activation, MG induced an increase in DNA strand breaks at concentrations ≥ 3 μg/mL, at which loss of viability ranged from 20% to 30%. The significance of the positive result observed at the 10 μg/mL dose is questionable due to a 70% loss of viability at this dose. After metabolic activation, DNA damage was observed at concentrations (15–20 μg/mL) where cell viability was reduced by less than 20%. In the same study, LMG tested at concentrations of 5–500 μg/mL did not induce DNA damage or loss of viability (Fessard et al., 1999).
MG-induced DNA strand breaks were reported also in Syrian hamster embryo (SHE) cells after exposure to 0.0125–0.1 μg/mL (Bose et al., 2005). In the same study, the authors observed MG-mediated cell cycle arrest and induction of apoptosis. Panandiker et al. (1994) showed an association of MG-induced DNA strand breaks with the formation of free radicals in SHE cells. Several studies showed that MG induces malignant transformation in vitro in SHE cells (Panandiker et al., 1993; Mahudawala et al., 1999). Au and Hsu (1979) reported no evidence of MG-induced chromosomal aberrations in vitro in cultured CHO cells.
In vivo genotoxicity studies for MG and LMG include chromosomal aberration and micronucleus assay, forward mutation assays, lacI and cII transgene mutation assays with rodents and 32P-postlabelling studies of liver DNA adduct formation. No increase in micronucleated erythrocytes was observed in bone marrow of mice administered a single dose of 37.5 mg/kg bw MG oxalate by gavage (Clemmensen et al., 1984).
In rat bone marrow cells, following three intraperitoneal injections of MG chloride at doses ranging from 1,094 to 8,750 mg/kg bw, a very small but significant increase in micronuclei frequency was observed only at a dose of 4,375 mg/kg bw (NTP, 2004). As the frequency of micronucleated polychromated erythrocytes (PCEs) was not significant at a dose of 8,750 mg/kg and no bone marrow toxicity was detected at this dose, the test in rats was judged to be negative overall. No increase in micronuclei was observed in peripheral blood normochromatic erythrocytes (NCEs) or PCEs of male and female mice after 28 days of exposure to MG chloride in feed (up to 1,200 mg/kg; approximately 250 mg/kg bw per day) (NTP, 2004).
MG also did not induce increase in micronucleated NCEs or PCEs in female Big Blue® B6C3F1 mice that received 450 mg/kg MG in the diet for 4 or 16 weeks (Mittelstaedt et al., 2004). On the contrary, Donya et al. (2012) reported a dose- and time-dependent increase in chromosomal aberration in bone marrow and spermatocytes in Swiss albino male mice treated daily by gavage with up to 543 mg MG/kg bw for 7, 14, 21 and 28 days. In the same study, the authors reported also dose- and time-dependent increase in sister chromatid exchanges in bone marrow and DNA fragmentation in liver cells. Induction of chromosomal aberrations, micronuclei formation and DNA damage in bone marrow have also been observed in Swiss albino female mice treated by gavage with 4 mg MG/kg bw for 30 days (Das et al., 2013).
A peripheral blood micronucleus test with LMG was performed in female B6C3F1/Nctr BR mice after 28 days of exposure to 290, 580, or 1,160 mg LMG/kg in feed. Significant increases in the frequency of micronucleated NCEs were observed in the 290 and 580 mg/kg groups. The increase of micronucleated PCEs was not significant (NTP, 2004). In female Big Blue® B6C3F1 mice that received 204 or 408 mg LMG/kg in the diet for 4 or 16 weeks, LMG did not induce an increase in micronucleated NCEs or PCEs (Mittelstaedt et al., 2004).
It has been shown that exposure to MG (100 or 600 mg/kg feed) for 28 days induces the formation of DNA adducts in liver of male F344 rats and female B6C3F1 mice (Culp et al., 1999). Under the same exposure conditions, LMG induced the formation of DNA adducts in rat, but not in mouse liver (Culp et al., 1999). A dose-related increase in DNA adduct levels in liver has been reported also in female Big Blue rats® fed a diet containing 9–543 mg LMG/kg for 4 weeks (Culp et al., 2002; Manjanatha et al., 2004). Because the results are based on 32P-postlabelling analysis, without further characterisation of the nature of adducts, the CONTAM Panel noted that these adducts are not necessarily MG/LMG metabolites bound to DNA.
When livers of female Big Blue® rats treated with 273 and 543 mg LMG/kg were analysed for lacI transgene mutations at 4, 16, and 32 weeks post-exposure, a significant increase (2.9-fold) in lacI mutant frequency was observed only in the livers of rats fed 543 mg LMG/kg for 16 weeks (Culp et al., 2002). However, molecular analysis of 80 of these liver lacI mutants revealed that 21% (17/80) were clonal in origin and that the majority (55/63) of the independent mutations were base pair substitutions involving guanine (G)-cytosine (C) to adenine (A)-thymine (T) transitions, similar to those found for control rats, suggesting that LMG is not a mutagen in the livers of female rats (Manjanatha et al., 2004). In the same female Big Blue® rats orally exposed to LMG, the analysis of the induction of HPRT mutations in lymphocytes also showed negative results (Manjanatha et al., 2004).
In another study, transgenic female Big Blue® B6C3F1 mice were given feed with MG (450 mg/kg) or LMG (204 and 408 mg/kg) for 4 and 16 weeks (Mittelstaedt et al., 2004). An increase in the liver cII transgene mutant frequency was observed after a 16-week treatment with 408 mg LMG/kg, indicating that LMG is an in vivo mutagen in transgenic female mouse liver. Molecular analyses of mutations from mice fed LMG had increased frequencies of G → T and A → T transversions. Statistical evaluation indicated that the spectrum of mutations for LMG-treated mice is significantly different from the controls. MG did not increase the mutation frequency of the cII transgene in the liver and neither MG nor LMG increased the HPRT mutation frequency in lymphocytes at either time point (Mittelstaedt et al., 2004).
| Test system | Cells/animals | Concentration/treatment | Result | Comment | Reference |
|---|---|---|---|---|---|
| In vitro | |||||
| Bacterial reverse mutation assay (Ames test) | Salmonella Typhimurium TA98, TA100, TA1535, TA1537 | 0.05–160 μg/plate (−S9) | Negative | Bacterial growth was inhibited at 1.28 μg/plate | Clemmensen et al. (1984) |
| 0.05–160 μg/plate (+S9) | Positive in TA98 at ≥ 6.4 μg/plate | No description on bacterial growth inhibitory concentration | |||
| S. Typhimurium TA98 | 10–150 μg/plate (+S9) | Positive at ≥ 30 μg/plate | Bacterial growth was inhibited at ≥ 100 μg/plate. Incidence of reverse mutation increased dose dependently at 20–70 μg/plate | ||
| S. Typhimurium TA98, TA100, TA1537 | Dose unspecified | Negative | Only without metabolic activation | Ferguson and Baguley (1988) | |
| S. Typhimurium TA97, TA98, TA100, TA102, TA104, TA1535 | 0.1–10 μg/plate (±S9) | Negative | Hamster- and rat-derived S9 mixes were used | NTP (2004) | |
| S. Typhimurium TA97a, TA98, TA100, TA102 | 0.01–10 μg/plate (±S9) | Negative | Bacterial growth was inhibited at ≥ 10 μg/plate in the absence of S9, except for TA98. In the presence of S9, the compound was tested up to 10 μg/plate, based on the results from preliminary cytotoxicity studies | Fessard et al. (1999) | |
| Mammalian cell forward mutation assay | CHO cells/HPRT | 0.001–0.05 μg/mL (−S9)/5 h | Negative | High cytotoxicity at ≥ 0.1 μg/mL. Positive result was obtained in one of the two trials at 0.01 μg/mL | Fessard et al. (1999) |
| 0.01–1 μg/mL (+S9)/5 h | Negative | ||||
| In vitro chromosomal aberrations | CHO cells | 20 μM (−S9) for 5 h | Negative | No information on cell viability or mitotic index. The test was performed only without metabolic activation | Au and Hsu (1979) |
| DNA strand breaks (Comet assay) | CHO cells | 1–10 μg/mL (−S9) | Positive ≥ 3 μg/mL | Cell survival was 80% at 3 μg/mL, 70% at 4 and 5 μg/mL and 30% at 10 μg/mL | Fessard et al. (1999) |
| 1–20 μg/mL (+S9) | Positive at ≥ 15 μg/mL | Cell survival was 80–90% at ≥ 15 μg/mL | |||
| SHE cells | 0.0125–0.1 μg/mL per 2 h | Positive at all conc. | Mild apoptosis at low doses | Bose et al. (2005) | |
| DNA strand breaks (alkaline elution assay) | SHE cells |
1 μg/mL for 2, 4, 6 and 24 h 0.1, 1 and 2 μg/mL for 4 h |
Positive |
Time-dependent increase in DNA strand break formation up to 4 h exposure then a decline was observed At 4 h exposure, significant increase was observed at 1 and 2 μg/mL |
Panandiker et al. (1994) |
| In vivo | |||||
| Micronucleus assay | Mouse bone marrow | Single p.o. dose of 37.5 mg/kg bw; sampling 24, 42, 66 h after | Negative | Clemmensen et al. (1984) | |
| Rat bone marrow | 1.094–8.750 mg/kg bw per day i.p. for 3 days | Negative | Significant increase was observed only at 4.375 mg/kg bw but not at other doses | NTP (2004) | |
| Female B6C3F1/NctrBR mouse peripheral blood | 25–1,200 mg/kg feed for 28 days (~ 5–250 mg/kg bw per day) | Negative | NTP (2004) | ||
| Female Big Blue® B6C3F1 mouse peripheral blood | 450 mg/kg feed for 4 or 16 weeks (~ 9 mg/kg bw per day) | Negative | Purity of 88% (major portion of the impurities (12%) was attributed to LMG and the remainder to demethylated forms of MG or LMG) | Mittelstaedt et al. (2004) | |
| Swiss albino female mice bone marrow | 100 μg/mice i.p. (~ 4 mg/kg bw per day) for 30 days | Positive |
Elevated ALT and AST activities; increased LPO and reactive aldehydes; depletion of GSH; modulation of antioxidative enzymes (GST, SOD, CAT, GPx, TrxR) Pretreatment with diphenylmethyl selenocyanate attenuated genotoxic effects |
Das et al. (2013) | |
| Chromosomal aberrations | Swiss albino female mice bone marrow and spermatocytes | 27–543 mg/kg bw per day by gavage for 7, 14, 21 and 28 days | Positive |
Time and dose-dependent increase of chromosomal aberrations in bone marrow and spermatocytes Depleted glutathione and increased free radical formation were observed |
Donya et al. (2012) |
| Swiss albino female mice bone marrow | 100 μg/mice i.p. (~ 4 mg/kg bw per day) for 30 days | Positive |
Elevated ALT and AST activities; increased LPO and reactive aldehydes; depletion of GSH; modulation of antioxidative enzymes (GST, SOD, CAT, GPx, TrxR) Pretreatment with diphenylmethyl selenocyanate attenuated genotoxic effects |
Das et al. (2013) | |
| DNA fragmentation (diphenylamine assay) | Swiss albino female mice liver cells | 27–543 mg/kg bw per day by gavage for 28 days | Positive | Donya et al. (2012) | |
| Forward mutation assay HPRT | Female Big Blue® B6C3F1 mouse spleen lymphocytes | 450 mg/kg feed for 4 or 16 weeks | Negative | Purity of 88% (major portion of the impurities (12%) was attributed to LMG and the remainder to demethylated forms of MG or LMG) | Mittelstaedt et al. (2004) |
| Transgenic animal mutation assay | Female Big Blue® B6C3F1 mouse liver cell cII mutations | 450 mg/kg feed for 16 weeks | Negative | The spectrum of cII mutations in treated mice was similar to that of control mice | Mittelstaedt et al. (2004) |
| 32P-postlabelling analysis | Female B6C3F1 mouse liver DNA | 100 and 600 mg/kg feed for 28 days | Positive | Significant increase of adducts at both concentrations | Culp et al. (1999) |
| Male F344 rat liver DNA | 100 and 600 mg/kg feed for 28 days | Positive | Significant increase of adducts at 600 mg/kg | Culp et al. (1999) | |
- ALT: alanine aminotransferase; AST: aspartate aminotransferase; bw: body weight; CAT: catalase; CHO: Chinese hamster ovary cells; conc.: concentrations; GPx: glutathione peroxidase; GSH: glutathione; GST: glutathione-S-transferase; HPRT: hypoxanthine–guanine phosphoribosyltransferase; i.p.: intraperitoneal; LMG: leucomalachite green; LPO: lipid peroxidation; MG: malachite green; p.o.: per os (orally); SHE: Syrian hamster embryo; SOD: superoxide dismutase; TrxR: thioredoxin reductase.
| Test system | Cells/animals | Concentration/treatment | Result | Comment | Reference |
|---|---|---|---|---|---|
| In vitro | |||||
| Bacterial reverse mutation assay (Ames test) | Salmonella Typhimurium TA97a, TA98, TA100 and TA102 | 10–2,000 μg/plate (±S9) | Negative | Bacterial survival decreased to 35–45% of that of the control at ≥ 1,000 μg/plate. Test substance precipitated at ≥ 500 μg/plate | Fessard et al. (1999) |
| Mammalian cell forward mutation assay | CHO cells/HPRT | 5–100 μg/mL (−S9) per 5 h | Negative | Cytotoxic at concentrations ≥ 500 μg/mL. Significant increase in HPRT mutations only at 75 μg/mL | Fessard et al. (1999) |
| 5–100 μg/mL (+S9) per 5 h | Negative | Significant increase in HPRT mutations only in one experiment at 5 μg/mL | Fessard et al. (1999) | ||
| DNA strand breaks (Comet assay) | CHO cells | 5–500 μg/mL (−S9) per h | Negative | Fessard et al. (1999) | |
| 25–300 μg/mL (+S9) per h | Negative | ||||
| In vivo | |||||
| Micronucleus assay | Female B6C3F1/NctrBR mouse peripheral blood | 290–1,160 mg/kg feed for 28 days | Positive at 290 and 580 mg/kg | Small increase observed at 1,160 mg/kg but not significant | NTP (2004) |
| Female Big Blue® B6C3F1 mouse peripheral blood | 204, 408 mg/kg feed for 4 or 16 weeks | Negative | Purity of 99% | Mittelstaedt et al. (2004) | |
| Forward mutation assay HPRT | Female Big Blue® B6C3F1 mouse spleen lymphocytes | 204, 408 mg/kg feed for 4 or 16 weeks | Negative | Mittelstaedt et al. (2004) | |
| Female Big Blue® rat spleen lymphocytes | 9–543 mg/kg feed for 4, 16, 32 weeks | Negative | Manjanatha et al. (2004) | ||
| Transgenic animal mutation assay | Female Big Blue® B6C3F1 mouse liver cell cII mutations | 204, 408 mg/kg feed for 4 and 16 weeks | Positive at 408 mg/kg | The spectrum of cII mutations in treated mice was significantly different from that of control mice | Mittelstaedt et al. (2004) |
| Female Big Blue® rat liver cell lacI mutations | 9–543 mg/kg feed for 32 weeks | Negative | Culp et al. (2002); Manjanatha et al. (2004) | ||
| 32P-postlabelling analysis | Female B6C3F1 mouse liver DNA | 96, 580 mg/kg feed for 28 days | Negative | Culp et al. (1999) | |
| Male F344 rat liver DNA | 96, 580 mg/kg feed for 28 days | Positive | Significant increase of adducts at 580 mg/kg | Culp et al. (1999) | |
| Female Big Blue® rat liver DNA | 9–543 mg/kg feed for 4 weeks | Positive | Dose-related significant increase of DNA adducts at ≥ 91 mg/kg | Culp et al. (2002) | |
- CHO: Chinese hamster ovary; HPRT: hypoxanthine–guanine phosphoribosyltransferase.
Conclusion
The results for genotoxicity studies with MG and LMG are summarised in Table 6. MG and LMG are not bacterial mutagens and also do not induce mutations in mammalian cells in vitro. However, it should be noted that because MG is highly toxic to bacteria it was tested only at low doses, with a few exceptions. In mammalian cells in vitro, MG induced DNA strand breaks, while LMG was inactive. A single report indicates that MG is not clastogenic in vitro. In vivo clastogenicity studies gave conflicting results for MG and LMG. Two short-term exposure bone marrow micronucleus assays with MG in mice and rats gave negative results, and two long-term exposure (28 days and 16 weeks) peripheral blood micronucleus assays in mice were also negative. Two other studies reported positive results for MG in the bone marrow micronucleus and chromosomal aberration assays in mice after long-term exposures (28 and 30 days). LMG was weakly positive in one long-term exposure peripheral blood micronucleus assay in mice, while negative results were reported in another study. Neither MG- nor LMG induced HPRT mutations in mice or rat spleen lymphocytes. Formation of DNA adducts was induced by MG in liver of rats and mice and by LMG in liver of rats, but not in liver of mice. However, characterisation of the nature of these adducts was not performed. No clear evidence for MG-induced cII transgene mutations in mouse liver was obtained. LMG increased mutation frequency of the cII transgene in mouse liver, with a unique mutational spectrum of increased G → T and A → T transversions. However, LMG did not induce lacI transgene mutations in rat liver.
JECFA concluded that MG has no genotoxic potential in conventional in vitro and in vivo assays and that LMG was negative in in vitro assays, but induced cII mutations in the liver of female Big Blue® B6C3F1 transgenic mice (FAO/WHO, 2009). However, at the time of that report the positive in vivo micronucleus and chromosomal aberration studies (Donya et al., 2012; Das et al., 2013) with MG were not available. ECHA (ECHA 2010a,b) evaluated the same data for MG and LMG as were used by JECFA. Based on the evidence that MG induced formation of DNA adducts in the liver of rats and mice and that LMG induced formation of DNA adducts in liver of rats and mutations in the liver of transgenic mice, ECHA presumed that MG and LMG may be potential in vivo somatic cell mutagens. Both compounds were classified as Muta.2 (suspected to cause genetic defects).
The CONTAM Panel concluded that: (a) the positive results obtained in the in vivo micronucleus tests in mice for MG and LMG, (b) the increased mutations in the cII transgene in mouse liver for LMG, and (c) the capacity of both MG and LMG to form DNA adducts in vivo provide evidence for considering MG and LMG as genotoxic in vivo.
| In vitro | In vivo | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bact. | Mammalian cells | Mice | Rats | |||||||||||||
| Mut | DSB | CA | Mut | DSB | CA | MN | Mut. HPRT | Mut. transgen | DNA adducts | DSB | CA | MN | Mut. HPRT | Mut. transgen | DNA adducts | |
| MG | N | P | N | N | P | P | N/P | N | N | P | – | – | N | – | – | P |
| LMG | N | N | – | N | – | – | N/(P) | N | P | N | – | – | – | N | N | P |
- CA: chromosomal aberration; DSB: DNA strand breaks; HPRT: hypoxanthine–guanine phosphoribosyltransferase; LMG: leucomalachite green; MG: malachite green; MN: micronucleus; Mut: mutations; Mut. trans: mutations in transgenic animals; N: negative result; P: positive result; (P): weakly positive; –: not tested.
3.3.2.7 Chronic toxicity and carcinogenicity
Groups of 48 female B6C3F1 mice were fed diets containing 0, 100, 225 or 450 mg MG/kg for 104 weeks (NTP, 2005; Culp et al., 2006). These dietary concentrations correspond to doses of approximately 0, 15, 33 or 67 mg/kg bw per day. The purity of MG was about 87%, with identified impurities of LMG (7.5%), N-desmethyl MG (3.8%) and N-desmethyl LMG (0.5%). There were no effects on body weight or on the survival rate of female mice, but absolute kidney weights were decreased in the mid- and high-dose groups. No treatment-related neoplasms were observed.
Groups of 48 female F344 rats were fed diets containing 0, 100, 300 or 600 mg MG/kg for 104 weeks (NTP, 2005; Culp et al., 2006), equivalent to average doses of 0, 7, 21 or 43 mg/kg bw per day. The purity of MG was about 87% (for impurities, see above). Body weights were decreased, particularly in the mid- and high-dose groups, being approximately 90% (mid dose) and 88% (high dose) of the control group weight at the end of the experimental period. In these groups, a small decrease in feed consumption was observed during the first year of the study compared to that of the control group. The survival rate was not affected. A small increase in the incidence of thyroid gland follicular cell adenoma or carcinoma was observed (0/46, 0/48, 3/47 and 2/46 for control, low-, mid- and high-dose groups, respectively). According to the NTP technical report (NTP, 2005), the incidence of thyroid gland follicular carcinoma was 0/46, 0/48, 2/47 and 1/46 for control, low-, mid- and high dose, respectively. The incidence of mammary gland carcinoma was increased in the high-dose group (2/48, 2/48, 1/48 and 5/48 for control, low-, mid- and high-dose groups, respectively). A dose-related increase in the incidence of hepatocellular adenomas was observed in female rats fed MG (1/48, 1/48, 3/48 and 4/48 for control, low-, mid- and high-dose groups, respectively), being significant only in the high-dose group.
Groups of 48 female B6C3F1 mice were fed diets containing 0, 91, 204 or 408 mg LMG/kg (purity about 99%) for 104 weeks (NTP, 2005; Culp et al., 2006). These dietary concentrations are equivalent to average doses of approximately 0, 13, 31 or 63 mg/kg bw per day. Body weight and survival rate were not affected by treatment with LMG, but a decrease in relative kidney weights was observed in all dose groups, however without a consistent dose–response relationship. The only neoplastic finding was a dose-related increase in the incidence of hepatocellular adenomas or carcinomas (3/47, 6/48, 6/47 and 11/47 for control, low-, mid- and high-dose groups, respectively), reported as significant in the high-dose group. The data reported by NTP (2005) indicated that hepatocellular adenomas formed the largest part of these tumours, and that only a low number of malignant liver tumours were found – the incidence of hepatocellular carcinomas alone was 0/47, 0/48, 1/47 and 2/47 for control-, low-, mid- and high-dose groups, respectively.
Male and female F344 rats (48 per sex) were fed diets containing 0, 91, 272 or 543 mg LMG/kg (purity about 99%) for 104 weeks (NTP, 2005; Culp et al., 2006). These dietary concentrations are equivalent to average doses of 0, 5, 15 or 30 mg/kg bw per day for males and 6, 17, or 35 mg/kg bw for females. Reduced body weights were found in both sexes, particularly in the mid- and high-dose groups, with body weights at termination of the experiment being approximately 92% (mid dose) and 87% (high dose) of the control group for males, and approximately 89% (mid dose) and 77% (high dose) of the control group for females. In mid- and high-dose males and females, a small decrease in feed consumption was observed throughout the study, compared to that by the control group. The survival rate was not affected. The absolute (and relative) liver weights of males were significantly increased in the mid- and high-dose groups. For females, only the relative liver weight was significantly increased in the mid- and high-dose groups. The incidence of hepatic cytoplasmic vacuolisation was increased in female rats (5/48, 5/48, 19/48, 22/48 for control, low-, mid- and high-dose groups, respectively). No further details on this effect were provided. In male and female rats, follicular cysts in the thyroid were found in the high-dose group, accompanied by an increase in relative thyroid weight. Both sexes also showed a low, not dose-related, increase in incidence of thyroid gland follicular cell adenomas or carcinomas (females 0/46, 1/46, 2/47, 1/48 and males 0/47, 2/47, 1/48, 3/46 for control, low-, mid- and high-dose groups, respectively). Female rats had an increased incidence of mammary gland adenomas or carcinomas: 0/48, 2/48, 3/48, 4/48 for control, low-, mid- and high-dose groups, respectively. The NTP (2005) report showed that the incidence of mammary gland adenomas was 0/48, 1/48, 1/48 and 2/48 and of mammary gland carcinomas 0/48/, 1/48, 2/48 and 2/48 for the control, low-, mid- and high-dose groups, respectively. Male rats had a dose-related increasing trend in interstitial cell adenomas of the testis (37/48, 42/47, 43/48, 45/47 for control, low-, mid- and high-dose groups, respectively).
Conclusion
The long-term studies evaluated above were all aimed at investigating the carcinogenic effects of MG and LMG and included a full histological assessment. Haematological and clinical chemical analyses were not included in these studies. MG was not carcinogenic in mice. In rats, a small, not dose-related, increase in the incidence of thyroid gland follicular adenomas and carcinomas and of mammary gland carcinomas was observed. Based on these observations in rats, the CONTAM Panel concluded that MG may be considered as carcinogenic.
LMG caused an increase in hepatocellular adenomas and a small increase in hepatocellular carcinomas in mice. In rats, LMG caused a small increase in the incidence of mammary gland carcinomas and of thyroid gland follicular cell adenomas or carcinomas (combined). Based on these observations in mice and rats, the CONTAM Panel concluded that LMG may be considered as carcinogenic.
3.3.3 Observations in humans
There is very limited information on the effects of MG/LMG in humans. Only one case report on intoxication with MG was identified.
A 3-year-old girl (17.3 kg bw) ingested about 45 mg MG (in the form of an aquarium product containing 0.075% MG) corresponding to a dose of about 2.6 mg/kg bw. She was admitted to the hospital half an hour after ingestion of the aquarium product with signs of cyanosis, including blue face, arms, hands, legs and feet. Blood analysis revealed that oxygen saturation was 47.4% and methaemoglobin level was 50.6%. She was treated with an infusion of methylene blue and, two and a half hours after ingestion of MG, the methaemoglobin level decreased to 6.5%. The child was observed for an additional 20 h, but symptoms did not return (Spiller et al., 2008).
3.3.4 Mode of action
MG is a conjugated iminium triphenylmethane dye available as the oxalate or hydrochloride salt. Due to its iminium structure, MG may behave as an electron-accepting/transferring compound leading to the formation of oxygen radical species that can affect a number of cellular processes (e.g. mitochondrial respiration) and result in GSH depletion (reviewed by Kovacic and Somanathan, 2014). In common with other electrophilic triphenylmethane dyes, it is also accepted that, at physiological pH, MG may form adducts with GSH, proteins and protein related nucleophiles (e.g. human serum albumin; see Section 3.3.1), possibly resulting in the inhibition of enzyme activities (Eldem and Özer, 2004; Tacal and Özer, 2004). In addition, MG and LMG may enter a CYP-mediated oxidative pathway yielding primary and secondary amine metabolites; in common with other aromatic amines, such metabolites may undergo further oxidation giving rise to unstable intermediates (-NHOH) and NO-derivatives (see Section 3.3.1), which have been linked with lipid peroxidation and DNA damage in cell cultures (Panandiker et al., 1992). Under in vitro conditions, LMG competitively inhibits TPO activity resulting in the impairment of tyrosine iodination and T4 formation (Doerge et al., 1998), with the potential for impairment of thyroid function. Chronic inhibition of thyroid hormone synthesis results in prolonged growth stimulus from TSH that can lead to thyroid follicular cell tumours. In vitro TPO-mediated LMG oxidation has also been demonstrated (see Section 3.3.1), so it cannot be excluded that the resulting reactive metabolites may display genotoxic activity at the thyroid level.
The MG-related cytotoxicity was correlated with effects on xenobiotic metabolising enzymes, lipid peroxidation and antioxidant status. SHE cells were exposed to increasing MG concentrations (0, 0.025, 0.05 and 0.1 μg/mL for 24 h, corresponding to 0, 0.0685, 0.137, and 0.274 μM, respectively). Compared to untreated controls, there was a concentration-related increase in both aryl-hydrocarbon hydroxylase (using benzo(a)pyrene as the substrate) and aminopyrine N-demethylase activities, reaching 181% and 295% of the control values, respectively. The rise in CYP-mediated monoxygenases was matched by a concurrent increase in lipid peroxidation (as measured by the amount of malondialdehyde produced), in catalase (CAT) activity, and in cytotoxicity expressed as relative plating efficiency. Results show that MG is able to induce CYP-dependent monooxygenases and they support the hypothesis that the observed cytotoxic effects might be related to an increase in the generation of reactive oxygen species (ROS), possibly including those derived from the (enhanced) oxidative biotransformation of MG itself (Panandiker et al., 1992). Further studies confirmed that exposure of SHE cells to MG-induced generation of free radicals (detected with electron spin resonance spectroscopy) and that it is associated with induction of lipid peroxidation and DNA damage (Panandiker et al., 1994). The study also showed that treatment of SHE cells with the antioxidant enzymes CAT and glutathione peroxidase (GPx) decreased MG-induced lipid peroxidation and DNA damage.
In mice, intraperitoneal exposure to 100 μg MG/mouse per day (equivalent to 4 mg/kg bw per day) for 30 days caused an increase in the levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST), indicators of liver injury, and an increase of hepatic lipid peroxidation, determined in terms of thiobarbituric acid reactive substances, an indicator of oxidative stress (Das et al., 2013). In the liver, MG caused depletion of reduced GSH and decrease in the activity of GST and of the antioxidant enzymes superoxide dismutase (SOD), CAT and GPx, which could lead to elevated levels of free radicals as indicated by increased lipid peroxidation. The CONTAM Panel noted that, under the same exposure conditions, MG also induced chromosomal aberrations and micronuclei in bone marrow and DNA strand breaks in lymphocytes (see Section 3.3.2.6). The same study showed that pre- or cotreatment of MG exposed mice with diphenylmethyl selenocyanate protects against MG-induced hepatotoxicity; this was reflected in lower levels of serum aminotransferases ALT and AST, lower levels of hepatic lipid peroxidation and higher levels and activities of detoxifying and antioxidative enzymes, as well as a moderate reduction in DNA damage compared to mice treated with MG alone. These results indicate a possible involvement of MG-induced oxidative stress in the mechanism of in vivo MG genotoxicity.
In isolated rat mitochondria, MG inhibited respiratory rates supported by succinate (a Complex II electron donor) while no inhibition of N,N,N8,N8-tetramethyl-p-phenylenediamine-supported respiration was observed, indicating that MG does not affect mitochondrial complex IV. MG promoted a dissipation of the mitochondrial membrane potential, accompanied by mitochondrial swelling, which was prevented by ethylene glycol tetraacetic acid (EGTA), Mg2+ and cyclosporin A, demonstrating that it induced mitochondrial permeability transition. This mitochondrial permeabilisation was induced by respiratory inhibition, attributable to cytochrome c release that was caused by the oxidation of NAD(P)H promoted by MG (Kowaltowski et al., 1999). These results indicate that MG could induce apoptosis via an intrinsic pathway.
Majeed et al. (2014) studied MG-induced cytotoxicity, genotoxicity, apoptosis and oxidative stress in vitro in fish cell lines (Channa striata kidney (CSK) and gill (CSG) cell lines). Cells were exposed to 0.001–10 μg/mL MG for 48 h. The viability was significantly reduced at concentrations ≥ 1 μg/mL. MG treatment for 48 h resulted in the distortion of mitochondrial shape and disorganisation of mitochondrial distribution in both CSK and CSG cell lines. The acridine orange/ethidium bromide staining showed that, at low concentration range (0.001–0.1 μg/mL), both early and late apoptotic cells occurred, while at higher concentrations the late apoptotic and necrotic cells were observed. A significant dose-dependent increase in DNA damage was observed at concentrations ≥ 0.1 μg/mL. Additionally, DNA electrophoretic mobility experiments, that were carried out to study the binding effect of MG to double-stranded DNA, showed that MG is capable of strong binding to linear double-stranded DNA, subsequently causing DNA degradation. In these cell lines, exposure to MG also induced significant increases in lipid peroxidation, and reductions in GSH and CAT activity, confirming the involvement of oxidative stress in MG-induced toxicity.
Induction of apoptosis and differential gene expression has been studied in human hepatoma HepG2 cells (Kim et al., 2008). Exposure of HepG2 cells to 0.0523, 0.867 and 1.202 μM MG concentrations, corresponding to the 10% inhibitory concentration (IC10), IC20 and IC30, respectively, for 48 h resulted in the induction of late apoptosis and necrosis in a dose-dependent manner. The gene expression pattern of selected apoptosis-related genes showed upregulation of tumour necrosis factor, p53, while expression of bax and caspase 3 were not changed. This result would indicate the involvement of an extrinsic (death receptor mediated) pathway in MG-induced apoptosis.
Pierrard et al. (2012) studied the mechanism of MG-induced toxicity by proteomic analysis (two-dimensional difference gel electrophoresis) in primary cultured peripheral blood mononuclear cells (PBMCs) from the Asian catfish, Pangasianodon hypophthalmus. The analysis showed that 109 proteins displayed significant changes in abundance in PBMC during 48 h exposure to MG. The data suggest that low concentrations of MG (1 and 10 ng/mL) could induce disturbances of mitochondrial metabolic functions, impairment of signal transduction and normal cell division, stimulation of DNA repair and disorganisation of the cytoskeleton.
A comparative in vitro cytotoxicity study of MG and LMG showed striking differences in their toxicity (Stammati et al., 2005). The compounds were tested in two human cell lines: HEp-2 cells derived from a human carcinoma of the larynx and Caco-2 cells derived from a human colon carcinoma. HEp-2 cells were exposed to 0.26, 1.4, 2.6 or 4.0 μM MG or 30, 150, 300 or 610 μM LMG and Caco-2 cells were exposed to 0.1, 1, 10, 25, 50 or 100 μM MG or 25, 50, 75 or 100 μM LMG for 24 h. Cytotoxicity was measured by neutral red uptake, total protein content, lactate dehydrogenase leakage, tetrazolium conversion and proliferation capability by the colony forming ability test (CFA). MG produced a clear dose-dependent inhibition of the viability of HEp-2 and CaCo-2 cells, whereas LMG showed only a small inhibition of CFA at the highest tested concentration in HEp-2 cells.
MG has been shown to induce morphological transformation of SHE cells exposed to 0.025, 0.05 and 0.1 μg/mL MG (Panandiker et al., 1993). It has been shown that in normal SHE cells, MG induces arrest in the G2/M phase of the cell cycle after 16 h of exposure, whereas in MG-treated transformed cells no such effect was observed indicating that in MG-transformed SHE cells the G2/M cell cycle checkpoint is abrogated (Rao et al., 1998). From the clone of MG-transformed cells, an immortalised MG-transformed cell line was established that showed increased DNA synthesis (determined by BrdU incorporation), increased levels of proliferating cell nuclear antigen (PCNA) and increased levels of p53 and bcl-2 proteins (Mahudawala et al., 1999). These MG-transformed cells exhibited higher expression and phosphorylation levels of vimentin, which has the role to link the nucleus to the plasma membrane possibly contributing to communication and transport between the cell surface and the nucleus (Mahudawala et al., 2000). A further study showed that in normal SHE cells MG induced time- and dose-dependent apoptosis, while in MG-transformed SHE cells induction of apoptosis by MG was marginal and was associated with over expression of p53 and bcl-2 proteins (Rao et al., 2000). Subcutaneous injection of these MG-transformed immortalised cells to nude mice resulted in the development of tumours that were transplantable and were histopathologically identified as sarcomas (Mahudawala et al., 1999).
The studies of changes in the cell signalling cascades during the MG-induced transformation of SHE cells, focusing on the mitogen-activated protein (MAP) kinase signal transduction pathway, showed that early changes associated with MG-induced malignant transformation of SHE cells are hyperphosphorylation of extracellular regulated kinases (ERK) and decreased phosphorylation of Jun N-terminal kinases (JNK), while no changes were observed in total ERK and JNK or in total and phosphorylated p38 (Bose et al., 2004, 2005). The study of the expression profile of these MAP kinases in MG-transformed cells, compared to normal SHE cells, showed decreased levels of phosphoactive ERK2 and JNK1 and 10-fold increased levels of phosphoactive p38 kinase (Bose et al., 2006).
Ashra and Rao (2006) studied MG-induced DNA damage, cell cycle arrest and the role of protein kinases Chk1, Chk2, Cdc2, Cdc25C, 14-3-3 and Cyclin B1 in normal and MG-transformed SHE cells. Exposure to MG caused DNA damage in both types of SHE cells, while cell cycle arrest in the G2/M phase was detected only in normal cells. Normal cells exposed to MG showed a decrease in Chk1 and an increase in Chk2, whereas in the MG-transformed cells an increase in phosphorylated Chk1 and a decrease in Chk2 were observed. Also, the levels of expression of Cdc25C, 14-3-3 and Cyclin B1 were elevated in normal cells, but decreased in MG-transformed cells. The study indicates that abrogation of G2/M checkpoint control during the transformation of SHE cells by MG is associated with elevated phosphorylation of Chk1 and decreased phosphorylation of Chk2 and decreased levels of Cyclin B1.
MG has been reported to act as a tumour promoter on the development of hepatic pre-neoplastic lesions (Fernandes et al., 1991; Rao and Fernandes, 1996). In male Wistar rats that were pretreated with the hepatocarcinogen N-nitrosodiethylamine (DEN; 200 mg/L in drinking water for a period of 1 month) followed by the treatment with MG (25, 50 and 100 mg/L in drinking water for 7 months) significantly higher numbers of GGT-positive foci, total GGT activity and the induction of glycogen-deficient islands were observed compared to animals treated with DEN alone. In subsequent mechanistic studies, it has been shown that in DEN-induced pre-neoplastic lesions, MG enhanced tyrosine phosphorylation, deregulated expression of cell cycle regulatory proteins cyclin D1 and B1 and their associated kinases (cdk4 and cdc2), elevated the level of PCNA, and stimulated DNA synthesis (Sundarrajan et al., 2000, 2001; Gupta et al., 2003).
MG (base, purity not given) was tested in an uterotropic assay for its estrogenic or antiestrogenic potency in groups of six ovariectomised C57BL/6J mice of 6 weeks of age. MG was administered orally by gavage at doses of 0, 3, 10, 30 or 100 mg/kg bw per day for 7 consecutive days at 24 h intervals, alone or in combination with ethynyl oestradiol (EO). Judged by the effect on uterus weight in combination with EE, MG exhibited a small, but significant, antiestrogenic effect at a dose of 100 mg/kg bw per day (Ohta et al., 2012).
Conclusion
MG has been shown to induce formation of ROS due to its behaviour as an electron-accepting/transferring compound. In addition, ROS can be formed from induction of CYP-dependent monooxygenases, as well as from oxidative biotransformation of MG itself. These types of effects have been shown to be related to cytotoxicity, DNA damage, induction of apoptosis, disturbances in cell cycle progression and MAP kinase signal transduction pathways and in vitro malignant transformation. In vivo, MG caused depletion of reduced GSH and decrease in the activity of GSTs and of the antioxidant enzymes SOD, CAT and GPx, which could lead to elevated levels of free radicals as indicated by increased hepatic lipid peroxidation. Therefore, the increased incidence of adenomas and carcinomas observed in MG/LMG-exposed rodents may be explained by increased induction of ROS. However, the formation of reactive metabolites causing adducts and mutations is also a possible mechanism for the observed genotoxic effects (see Section 3.3.2.6).
LMG has been shown to inhibit TPO in vitro, being also biotransformed by TPO into N-demethylated derivatives that could, in turn, generate reactive metabolites. This might explain the reported increased incidence of thyroid gland follicular cell adenoma or carcinoma in female rats in the carcinogenicity study by NTP (2005).
3.3.5 Consideration of critical effects, dose–response assessment and derivation of a health-based guidance value
3.3.5.1 Neoplastic effects
For both MG and LMG, long-term carcinogenicity studies are available (see Section 3.3.2.7). The CONTAM Panel concluded that MG may be considered as carcinogenic in rats and LMG in both mice and rats. No information was identified on the carcinogenicity of MG or LMG in humans. For the selection of the reference point for neoplastic effects, the CONTAM Panel focused on those organs in which malignant tumours had been observed.
MG induced thyroid gland follicular cell adenomas or carcinomas and mammary gland carcinomas in female F344 rats, but in female B6C3F1 mice no treatment-related neoplasms were observed (NTP, 2005; Culp et al., 2006). Based on positive results in the in vivo micronucleus tests in mice and the capacity to form DNA adducts in vivo, the CONTAM Panel considered MG to be genotoxic in vivo. Therefore, MG may be regarded as a substance which is genotoxic and carcinogenic.
LMG induced hepatocellular adenomas or carcinomas in female B6C3F1 mice and, in female F344 rats, induced thyroid gland follicular cell adenomas or carcinomas and mammary gland adenomas or carcinomas. Based on positive results in the in vivo micronucleus tests in mice, positive results in cII transgene mutations in mouse liver, and the capacity to form DNA adducts in vivo, the CONTAM Panel considered LMG to be genotoxic in vivo. Therefore, LMG may be regarded as a substance which is genotoxic and carcinogenic.
Based on these conclusions regarding the genotoxicity and carcinogenicity of MG and LMG, the CONTAM Panel concluded that the derivation of a health-based guidance value (HBGV) for these compounds is not appropriate, and decided to apply an MOE approach for the risk characterisation.
- LMG induced hepatocellular adenomas and carcinomas in female mice. The CONTAM Panel noted the low increase (< 10%) of tumour incidence in the treated groups compared to the control group. The EFSA Scientific Committee indicated that a BMR of 10% is the ‘lowest statistically significant increased incidence that can be measured in most studies, and would normally require little or no extrapolation outside the observed experimental data’ (EFSA, 2005a). Therefore, the CONTAM Panel concluded that using the data on carcinomas alone would result in an increase of the uncertainty regarding the reference point and considered the data not to be suitable for calculating a BMDL10. Instead, the CONTAM Panel used the combined data on adenomas or carcinomas in female mice for BMD analysis (see Appendix C, Section C.1). Based on these data, the CONTAM Panel derived a lowest BMDL10 of 13.1 mg/kg bw per day.
- MG and LMG induced mammary gland adenomas and carcinomas in rats. The CONTAM Panel noted the low increase (< 10%) of tumour incidence in the treated groups compared to the control group considering both substances separately or combined. For the same reason as mentioned above, the CONTAM Panel concluded that using the data on mammary gland adenomas and carcinomas would result in an increase of the uncertainty regarding the reference point and considered the data not to be suitable for calculating a BMDL10.
- The data on thyroid gland follicular cell adenomas and carcinomas induced by MG and LMG in rats were not used for BMD analysis because of the lack of a consistent dose–response relationship.
| MG | LMG | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female F344 rats | Female B6C3F1 mice | Female F344 rats | Male F344 rats | |||||||
| Dose (mg/kg bw per day) | Mammary gland carcinomas | Thyroid gland follicular cell adenomas and carcinomas | Dose (mg/kg bw per day) | Hepatocellular carcinomas | Hepatocellular adenomas and carcinomas | Dose (mg/kg bw per day) | Mammary gland adenomas and carcinomas | Thyroid gland follicular cell adenomas and carcinomas | Dose (mg/kg bw per day) | Thyroid gland follicular cell adenomas and carcinomas |
| 0 | 2/48a | 0/46 | 0 | 0/47 | 3/47 | 0 | 0/48 | 0/46 | 0 | 0/47 |
| 7 | 2/48 | 0/48 | 13 | 0/48 | 6/48 | 6 | 2/48 | 1/46 | 5 | 2/47 |
| 21 | 1/48 | 3/47 | 31 | 1/47 | 6/47 | 17 | 3/48 | 2/47 | 15 | 1/48 |
| 43 | 5/48 | 2/46 | 63 | 2/47 | 11/47 | 35 | 4/48 | 1/48 | 30 | 3/46 |
- bw: body weight.
- a Number of animals with tumours/total number of animals.
The CONTAM Panel selected the BMDL10 value of 13 mg/kg bw per day (rounded figure) derived for LMG. As both the RPA and the data on occurrence of MG and LMG in fish and crustaceans are expressed for MG/LMG, and consequently, the exposure is estimated for MG/LMG, the CONTAM Panel used the selected BMDL10 value as a reference point for the neoplastic effects of MG/LMG.
The CONTAM Panel noted that JECFA also selected the incidence of hepatocellular adenomas and carcinomas (combined) in female mice induced by LMG as the pivotal effect for the risk assessment. JECFA calculated BMDL10 values ranging from 18.5 to 31.2 mg/kg bw per day and selected a BMDL10 of 20 mg LMG/kg bw per day as the reference point for the risk characterisation of neoplastic effects. The CONTAM Panel also noted that JECFA used 15 mg/kg bw per day as the dose for the low-dose group, while in the current risk assessment a dose of 13 mg/kg bw per day, which is the dose reported in the NTP study, is used (NTP, 2005).
3.3.5.2 Non-neoplastic effects
For the evaluation of non-neoplastic effects, 28-day, long-term (104 weeks) and reproductive and developmental toxicity studies are available for both MG and LMG (see Section 3.3.2).
- The data on post-implantation loss and skeletal abnormalities caused by LMG (Wan et al., 2011) in rats were considered not suitable for deriving a reference point due to the maternal toxicity observed at doses causing fetal toxicity. In addition, there is a lack of separate data for each litter and therefore a BMD analysis cannot be adjusted for litter effects. However, the CONTAM Panel identified from these data a NOAEL of 10 mg/kg bw per day.
- Increases in relative liver weight observed in 28-day and long-term studies caused by MG and LMG in rats were not considered an appropriate endpoint due to the observed decrease in body weight.
- Increases in absolute liver weight in rats observed in a 28-day study with MG and in a long-term study with LMG were considered suitable for BMD analysis (see Appendix C, Sections C.2 and C.3). BMDL05 values of 5.9 and 7.2 mg/kg bw per day, respectively, were derived.
- Decreases in body weight observed in a long-term study with LMG in male and female rats and with MG in female rats were considered suitable for BMD analysis (see Appendix C, Section C.4). A lowest BMDL05 value (for female rats) of 5.8 mg/kg bw per day was derived for LMG.
- In a 28-day study with male rats, LMG increased the incidence of hepatocyte cytoplasmic vacuolisation in a dose-related manner in all treated groups. In a similar study with MG, the same effect was found in male rats, but only at higher dose levels. In long-term studies, hepatocyte cytoplasmic vacuolisation was only observed in female rats in the two highest dose groups, but without a clear dose–response relationship. The CONTAM Panel noted that this effect indicated that the liver was a target organ for toxicity, but because information on the nature and the severity of the effect was lacking, its relevance could not be judged and the data were considered not to be suitable for deriving a reference point.
- The CONTAM Panel noted an increase in reticulocytes and a small decrease in the levels of haemoglobin, haematocrit and erythrocytes in a 28-day study with MG in male and female mice treated at high doses (from 120 mg/kg bw per day onwards for females and at 220 mg/kg bw per day for males), which indicate mild anaemia. Because the biological relevance of such small effects is unclear, the CONTAM Panel considered the data not to be suitable for deriving a reference point.
- Overall for MG, a NOAEL of 9.4 mg/kg bw per day was identified for haematological effects in rats.
Based on the effect of MG on liver weight and of LMG on body weight, the CONTAM Panel selected the BMDL05 value of 6 mg/kg bw per day (rounded figure), as a reference point for the non-neoplastic effects of MG/LMG. The CONTAM Panel noted that this value is higher than the acute dose that caused adverse effects in a case of intoxication of an infant. However, the composition of the product was not reported and this is further addressed in Section 3.5.
3.4 Risk characterisation
Exposure of fish and crustaceans to MG results in residues of MG and its metabolite LMG occurring in tissue. Only limited occurrence data on MG/LMG in food were available for this opinion (see Section 2.1.1). The CONTAM Panel concluded that these data are not suitable to carry out a reliable human dietary exposure assessment. Therefore, the CONTAM Panel cannot characterise the risk of actual exposure to MG/LMG.
3.4.1 Evaluation of whether a reference point for action of 2 μg/kg for the sum of MG and LMG is adequate to protect human health
To evaluate whether or not an RPA of 2 μg/kg for the sum of MG and LMG is adequate to protect human health, the CONTAM Panel calculated the hypothetical chronic dietary exposure to MG/LMG from all type of fish, fish products and crustaceans, with the exclusion of aquatic molluscs, assuming that all products would be contaminated at the level of the RPA.
The CONTAM Panel selected a BMDL10 value of 13 mg/kg bw per day, derived for LMG, as reference point for the risk characterisation of neoplastic effects caused by MG/LMG.
The median hypothetical chronic dietary exposure to MG/LMG across dietary surveys for the average consumer would be 1.2 and 0.6 ng/kg bw per day for toddlers (the highest exposed population group) and adults, respectively. The minimum and maximum hypothetical chronic dietary exposures across dietary surveys for the average consumer would be 0.5 and 5.0 ng/kg bw per day, respectively, for toddlers and 0.2 and 1.9 ng/kg bw per day, respectively, for adults (see Table 1).
When comparing the median hypothetical chronic dietary exposure to MG/LMG across dietary surveys for the average consumer with the BMDL10 of 13 mg/kg bw per day for neoplastic effects, the MOE would be about 1.1 × 107 for toddlers and 2.2 × 107 for adults. For the minimum and maximum hypothetical chronic dietary exposures across dietary surveys for the average consumer, the MOEs for toddlers would be about 2.6 × 107 and 2.6 × 106, respectively, and for adults would be about 6.6 × 107 and 6.9 × 106, respectively.
As there is a concern that high and frequent consumers of fish might have elevated levels of MG/LMG dietary exposure, a scenario for these consumers was elaborated. For high and frequent fish consumers, the median hypothetical chronic dietary exposure to MG/LMG across dietary surveys would be 6.1 and 3.8 ng/kg bw per day for toddlers and adults, respectively. The minimum and maximum hypothetical chronic dietary exposures across dietary surveys would be 2.6 and 11.8 ng/kg bw per day, respectively, for toddlers and 1.3 and 5.2 ng/kg bw per day, respectively, for adults (see Table 2).
When comparing, for high and frequent fish consumers, the median hypothetical chronic dietary exposure to MG/LMG across dietary surveys with the BMDL10 for neoplastic effects, the MOE would be about 2.1 × 106 for toddlers and 3.4 × 106 for adults. For the minimum and maximum hypothetical chronic dietary exposures across dietary surveys, the MOEs for toddlers would be about 5.0 × 106 and 1.1 × 106, respectively, and for adults would be about 1.0 × 107 and 2.5 × 106, respectively.
For substances that are both genotoxic and carcinogenic, the EFSA Scientific Committee proposed that an MOE of 10,000 or higher, if based on the BMDL10 from an animal carcinogenicity study, would be of low concern from a public health point of view (EFSA, 2005a). Considering that the calculated MOEs for carcinogenicity would be of the order of 106 and higher, they do not indicate a health concern.
For non-neoplastic effects, the CONTAM Panel identified a BMDL05 of 6 mg/kg bw per day for the effect on body weight and liver weight caused by MG/LMG.
When comparing the median hypothetical chronic dietary exposure to MG/LMG across dietary surveys for the average consumer with the BMDL05 of 6 mg/kg bw per day for non-neoplastic effects, the MOE would be about 4.8 × 106 for toddlers and 9.7 × 106 for adults. For the minimum and maximum chronic hypothetical dietary exposures across dietary surveys for the average consumer, the MOEs for toddlers would be about 1.2 × 107 and 1.2 × 106, respectively, and for adults would be about 2.9 × 107 and 3.1 × 106, respectively.
When comparing, for high and frequent fish consumers, the median hypothetical chronic dietary exposure to MG/LMG across dietary surveys with the BMDL05 for non-neoplastic effects, the MOE would be about 9.5 × 105 for toddlers and 1.5 × 106 for adults. For the minimum and maximum hypothetical chronic dietary exposures across dietary surveys, the MOEs for toddlers would be about 2.2 × 106 and 4.9 × 105, respectively, and for adults would be about 4.5 × 106 and 1.1 × 106, respectively.
The CONTAM Panel noted that MOEs larger than 100 are often considered to be of low concern for threshold effects (FAO/WHO, 2009). Considering that the calculated MOEs for the effects on body weight and on liver weight would be of the order of 105 or higher, they are considered sufficiently large and do not indicate a health concern for non-neoplastic effects.
Overall, the CONTAM Panel concluded that it is unlikely that exposure to food contaminated with MG/LMG at or below the RPA of 2 μg/kg represents a health concern.
3.5 Uncertainty analysis
The CONTAM Panel concluded that the lack of representative occurrence data on MG/LMG in food precludes a reliable human dietary exposure assessment. The CONTAM Panel made exposure scenarios by calculating the hypothetical human chronic dietary exposure using the current RPA of 2 μg/kg for all types of fish, fish products and crustaceans (excluding aquatic molluscs) as a hypothetical occurrence value for average consumers and for high and frequent consumers of fish. This is in agreement with the request to evaluate if the RPA is low enough to protect human health. These scenarios represent highly unlikely situations and, as they consider that all selected food products are simultaneously contaminated with MG/LMG at the RPA, are likely to grossly overestimate the exposure.
The risks from MG and LMG are evaluated based on their potential genotoxic and carcinogenic properties. The data on the genotoxicity of MG and LMG contain a number of contradictions. Both compounds were negative in standard in vitro genotoxicity tests. In vivo, both compounds induced DNA adducts that were detected by the 32P-postlabelling technique, without further characterisation of the nature of these adducts. In the in vivo genotoxicity tests, only LMG showed a weak mutagenic effect. There were also indications for clastogenic effects of MG and LMG but, overall, the evidence is rather weak. In addition, it is not clear whether the mechanism of genotoxicity is due to the formation of specific MG- and LMG-induced DNA adducts or via the generation of ROS.
The CONTAM Panel concluded that MG and LMG may be considered as carcinogenic and genotoxic compounds. MG/LMG did cause liver, mammary and thyroid tumours, but for some of these tumour types only small (less than 10% of the animals) and/or non-dose-related increases of the incidence were observed. Reliable dose–response modelling was only feasible when combining the incidence of hepatocellular carcinomas and adenomas. However, only some of the adenomas may develop into carcinomas. Furthermore, there are indications that tumours might develop because of the tumour promoting capacity of MG. The relatively low tumour incidence adds to the uncertainty of the BMD-modelling. Overall, considering MG and LMG as carcinogenic and genotoxic compounds as well as including adenomas in the modelling may result in an overestimation of the risk.
The CONTAM Panel identified only one case report indicating that a single exposure to malachite green at a dose of about 2.6 mg/kg bw caused methaemoglobinaemia. This dose is below the selected BMDL05 of 6 mg/kg bw per day for non-neoplastic effects. However, the CONTAM Panel noted that MG was ingested in the form of an aquarium product containing only 0.075% MG and that the composition of the product was not reported. The presence of other compounds causing methaemoglobinaemia cannot be excluded and, therefore, the study cannot be used for the risk assessment.
N-demethylated metabolites with potential genotoxic activity have been detected in tissues of rats and fish exposed to MG/LMG. These metabolites are not included in the RPA and this may result in an underestimation of the risk for humans.
No information regarding the potential formation of degradation products of MG/LMG during food processing was identified. This lack of information adds to the uncertainty.
Overall, the CONTAM Panel considered that the impact of the uncertainties on the risk assessment of human exposure to MG/LMG through the consumption of food is substantial. Both the exposure scenario and the hazard characterisation are overestimated and therefore the approach taken is likely to overestimate the risk.
4 Conclusions
4.1 General
- Malachite green (MG) is a triphenylmethane dye that is used to colour materials such as paper and textiles.
- MG has been widely used as a therapeutic agent for fish and crustaceans due to its antifungal and antiprotozoal activity. However, in the European Union, MG is not registered for use in food-producing animals.
- Residues of the parent compound are less persistent than those of the reduced metabolite leucomalachite green (LMG).
4.1.1 Analytical methods
- Screening and confirmatory methods are usually designed to measure both substances independently or to measure both substances as MG, following oxidation of LMG to MG.
- Screening methods for MG/LMG include immunoassays (ELISA, RNA aptamer assay, biosensors), electrochemiluminescence and HPLC-UV/Vis and/or fluorescence detection, with detection capability values typically ≤ 0.5 μg/kg.
- LC–MS (typically tandem mass spectrometry) is the method of choice for confirmatory analysis of MG/LMG in fish and crustaceans.
4.2 Occurrence/exposure
- Data on occurrence of MG/LMG in food were extracted from the European Commission database on monitoring of veterinary medicinal product residues and other substances in fish, fish products and crustaceans for the years 2002–2014. There were 548 targeted samples reported as non-compliant. Data from Norway for the years 2006–2014 were also considered.
- Data were extracted from the RASFF database for the years 2002–2014. There were 135 notification events reported for MG/LMG. The notifications covered the product categories fish and fish products, crustaceans and products thereof, farmed fish and products thereof (other than crustaceans and molluscs), and wild-caught fish and products thereof (other than crustaceans and molluscs).
- The CONTAM Panel concluded that data extracted from the European Commission database and the RASFF database were not suitable to carry out a reliable human dietary exposure assessment.
- The CONTAM Panel calculated the hypothetical human dietary exposure considering as an occurrence value the RPA of 2 μg/kg for all types of fish, fish products and crustaceans.
- The mean hypothetical chronic dietary exposure across the different European dietary surveys and age classes, would range from a minimum of 0.1 ng/kg bw per day for infants, elderly and very elderly to 5.0 ng/kg bw per day for toddlers.
- As there is a concern that high and frequent fish consumers might have elevated MG/LMG dietary exposure, the exposure for these consumers was considered separately. The 95th percentile hypothetical chronic dietary exposure in fish consumers only, across the different European dietary surveys and age classes, would range from a minimum of 1.3 ng/kg bw per day for adults to a maximum of 11.8 ng/kg bw per day for toddlers.
4.3 Food processing
- Concentrations of MG and LMG residues in fish muscle are reduced when the muscle is subjected to cooking conditions, such as boiling, baking and microwaving, or when stored under refrigeration, freezing or repeated freezing/thawing conditions. The concentrations of LMG, generally, are reduced to a lesser extent than those of MG.
4.4 Hazard identification and characterisation
4.4.1 Toxicokinetics
- There is scant information about MG and LMG kinetics in mammalian species and no data were identified for humans.
- In rats, MG is rapidly absorbed and excreted mainly by the faecal route. Available data indicate that MG undergoes biliary excretion, possibly as a GSH adduct.
- In orally dosed rats and mice, MG is reduced to LMG and both undergo hepatic sequential N-demethylation.
- An N-oxide derivative, resulting from the oxidative biotransformation of an N-demethylated metabolite, has been identified in liver extracts from LMG-administered mice.
- In fish, MG is rapidly absorbed and extensively biotransformed to LMG, which is stored in tissues and slowly excreted. MG N-oxide and MG N-demethylated derivatives were also detected in edible fish tissues.
- Persistence of MG and LMG residues in fish depends on fish species and size, MG exposure concentration and duration, and environmental conditions including temperature and pH. In fish, MG was detected up to about 2 months and LMG was detected up to about 9 months after cessation of exposure.
- In crustaceans (shrimp), residues of MG/LMG were no longer detectable 9 days following treatment.
4.4.2 Toxicity studies
- In 28-day toxicity tests, MG caused haematological effects in mice and rats and an increase in liver weight in male and female rats. The overall NOAEL, based on haematological effects, in female rats was 9.4 mg/kg bw per day.
- For LMG, in 28-day toxicity tests, an increase in liver weight was found in male rats. Hepatocyte vacuolisation was found at all doses tested, including the lowest dose of 30 mg/kg bw per day. Therefore, a NOAEL was not identified for LMG regarding hepatotoxicity.
- For LMG, a NOAEL of 10 mg/kg bw per day for fetal toxicity in rats was identified. The CONTAM Panel noted, however, that these effects were observed at doses also causing maternal toxicity.
- The positive results obtained in the in vivo micronucleus tests in mice for MG and LMG, the increased mutations in the cII transgene in mouse liver for LMG, and the capacity of both MG and LMG to form DNA adducts in vivo provide evidence for considering MG and LMG as genotoxic in vivo.
- In long-term studies, an increase in liver weight was observed in male rats. LMG caused an increase in relative thyroid weight in male and female rats.
- MG was not carcinogenic in mice. However, MG induced a small, not dose-related, increase in the incidence of thyroid gland follicular adenomas and carcinomas and of mammary gland carcinomas in female rats. The CONTAM Panel concluded that MG may be considered as carcinogenic.
- LMG caused an increase in hepatocellular adenomas and a small increase in hepatocellular carcinomas in mice. In rats, LMG caused a small increase in the incidence of mammary gland carcinomas and of thyroid gland follicular cell adenomas or carcinomas (combined). The CONTAM Panel concluded that LMG may be considered as carcinogenic.
4.4.3 Observations in humans
- Only one report was identified, describing a case of methaemoglobinaemia in a 3-year-old girl following a single exposure to MG (about 2.6 mg/kg bw) in the form of an aquarium product containing 0.075% MG. However, the composition of the product was not reported.
4.4.4 Mode of action
- MG has been shown to induce formation of ROS due to its behaviour as an electron-accepting/transferring compound. In addition, ROS can be formed from induction of CYP-dependent monooxygenases, as well as from oxidative biotransformation of MG itself.
- MG-mediated formation of ROS has been associated with MG-induced cytotoxicity, DNA damage, apoptosis, disturbances in cell cycle progression and MAP kinase signal transduction pathways and in vitro malignant transformation.
- In vivo, MG caused depletion of GSH and a decrease in the activity of GSTs and antioxidant enzymes that could lead to elevated levels of free radicals as indicated by increased hepatic lipid peroxidation.
- LMG has been shown to inhibit TPO in vitro. It can also be biotransformed by TPO into N-demethylated derivatives which could, in turn, generate reactive metabolites. This may explain the effects observed in the thyroid.
4.4.5 Considerations of critical effects, dose–response modelling and possibilities for derivation of a health-based guidance value
- Because MG and LMG may be regarded as substances that are genotoxic and carcinogenic, the derivation of a health-based guidance value is not appropriate. A BMDL10 for hepatocellular adenomas and carcinomas in female mice of 13 mg/kg bw per day was selected as a reference point for neoplastic effects of MG/LMG.
- Based on the effect of MG on liver weight and of LMG on body weight, the CONTAM Panel selected the BMDL05 value of 6 mg/kg bw per day as a reference point for the non-neoplastic effects of MG/LMG.
4.5 Risk characterisation
- Due to the limited occurrence data for MG/LMG, no reliable human dietary exposure assessment could be carried out and, therefore, the CONTAM Panel could not characterise the risk.
4.5.1 Evaluation of whether an RPA of 2 μg/kg for the sum of MG and LMG is adequate to protect public health
- For the average consumer, the median hypothetical chronic dietary exposure to MG/LMG across dietary surveys would result in margins of exposure (MOE) for neoplastic effects of about 1.1 × 107 for toddlers and 2.2 × 107 for adults, and in MOEs for non-neoplastic effects of about 4.8 × 106 for toddlers and 9.7 × 106 for adults. The CONTAM Panel considered that these MOEs for neoplastic and non-neoplastic effects are sufficiently large and do not indicate a health concern.
- For high and frequent fish consumers, the median hypothetical chronic dietary exposure to MG/LMG across dietary surveys would result in MOEs for neoplastic effects of about 2.1 × 106 for toddlers and 3.4 × 106 for adults, and in MOEs for non-neoplastic effects of about 9.5 × 105 for toddlers and 1.5 × 106 for adults. The CONTAM Panel considered that these MOEs for neoplastic and non-neoplastic effects are sufficiently large and do not indicate a health concern.
- Overall, the CONTAM Panel concluded that it is unlikely that exposure to food contaminated with MG/LMG at or below the RPA of 2 μg/kg represents a health concern.
5 Recommendations
- Data regarding the occurrence of additional metabolites in fish and crustaceans should be generated.
- Further information on the fate of MG and LMG during food processing should be developed.
- Knowledge concerning the toxicokinetics and bioavailability of MG/LMG using human in vitro models should be improved.
- Information regarding the potential genotoxicity of the N-demethylated metabolites should be developed.
- The DNA adducts and GSH adducts observed in rodents should be characterised.
Documentation provided to EFSA
- Law FCP, 1994. Total Residue Depletion and Metabolic Profile of Selected Drugs in Trout. US Food and Drug Administration, Contract number 223-90-7016. Study conducted at Simon Fraser University, Burnby, British Columbia, Canada. Submitted by the United States Food and Drug Administration.
Notes
References
Abbreviations
-
- (P)
-
- weakly positive
-
- A
-
- Adenine
-
- ADI
-
- acceptable daily intake
-
- AFC Panel
-
- EFSA Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food
-
- AFSSA
-
- l'Agence française de sécurité sanitaire des aliments
-
- ALT
-
- alanine aminotransferase
-
- ANSES
-
- Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail
-
- APCI–MS
-
- atmospheric pressure chemical ionisation–mass spectrometry
-
- AST
-
- aspartate aminotransferase
-
- bw
-
- body weight
-
- BfR
-
- German Federal Institute for Risk Assessment
-
- BMD
-
- benchmark dose
-
- BMD05
-
- benchmark dose for a response of 5% extra risk
-
- BMDL
-
- benchmark dose lower confidence limit
-
- BMDL05
-
- lower 95% confidence limit for a benchmark response of 5% extra risk
-
- BMDL10
-
- lower 95% confidence limit for a benchmark response of 10% extra risk
-
- BMDU05
-
- upper 95% confidence limit for a benchmark response of 5% extra risk
-
- BMR
-
- benchmark response
-
- C
-
- cytosine
-
- CA
-
- chromosomal aberration
-
- CAS
-
- Chemical Abstracts Service
-
- CAT
-
- catalase
-
- CCα
-
- decision limit
-
- CCβ
-
- detection capability
-
- CFA
-
- colony forming ability test
-
- CHO
-
- Chinese hamster ovary
-
- CLP
-
- classification, labelling and packaging
-
- CONTAM Panel
-
- EFSA Scientific Panel on Contaminants in the Food Chain
-
- CSG
-
- Channa striata gill
-
- CSK
-
- Channa striata kidney
-
- CVMP
-
- Committee for Veterinary Medicinal Products
-
- CYP
-
- cytochrome P450
-
- DAD
-
- diode-array detection
-
- DDQ
-
- 2,3-dichloro-5,6-dicyano-1,4-benzoquinone
-
- DEN
-
- N-nitrosodiethylamine
-
- DSB
-
- DNA strand breaks
-
- DTU
-
- Technical University of Denmark
-
- ECHA
-
- European Chemicals Agency
-
- EEA
-
- European Economic Area
-
- EGTA
-
- ethylene glycol tetraacetic acid
-
- ELISA
-
- enzyme-linked immunosorbent assays
-
- EMA
-
- European Medicines Agency
-
- EO
-
- ethynyl oestradiol
-
- EPI
-
- estimation program interface
-
- ERK
-
- extracelluar regulated kinases
-
- ESI
-
- electrospray ionisation
-
- EURL
-
- European Union Reference Laboratory
-
- FAO
-
- Food and Agriculture Organization
-
- FAPAS
-
- Food Analysis Performance Assessment Scheme
-
- FSANZ
-
- Food Standards Australia New Zealand
-
- FSC
-
- Food Safety Commission
-
- FW
-
- freshwater
-
- G
-
- guanine
-
- GGT
-
- gamma-glutamyl transferase
-
- GPx
-
- glutathione peroxidase
-
- GSH
-
- glutathione
-
- GST
-
- glutathione-S-transferase
-
- HBGV
-
- health-based guidance value
-
- HPRT
-
- hypoxanthine–guanine phosphoribosyltransferase
-
- HPLC
-
- high performance liquid chromatography
-
- i.p.
-
- intraperitoneal
-
- IAC
-
- immunoaffinity chromatography
-
- IC
-
- inhibitory concentration
-
- IC50-D
-
- concentration causing 50% reduction of differentiation
-
- IC50-P
-
- concentration causing 50% inhibition of viability/proliferation
-
- ID-LC/MS
-
- isotope dilution liquid chromatography/mass spectrometry
-
- JECFA
-
- Joint FAO/WHO Expert Committee on Food Additives
-
- JNK
-
- Jun N-terminal kinases
-
- K i
-
- inhibition constant
-
- LC–MS
-
- liquid chromatography–mass spectrometry
-
- LC–MS/MS
-
- liquid chromatography–tandem mass spectrometry
-
- LD50
-
- median lethal dose
-
- LMG
-
- leucomalachite green
-
- LOD
-
- limit of detection
-
- LOEL
-
- lowest-observed-effect level
-
- Log Kow
-
- octanol/water partition coefficient
-
- LOQ
-
- limit of quantification
-
- LPO
-
- lipid peroxidation
-
- MAP
-
- mitogen activated protein
-
- MG
-
- malachite green
-
- MIP
-
- molecularly imprinted polymer
-
- MN
-
- micronucleus
-
- MOE
-
- margin of exposure
-
- MRL
-
- maximum residue limit
-
- MRPL
-
- minimum required performance limit
-
- Mut
-
- mutations
-
- N
-
- negative result
-
- NADPH
-
- nicotinamide adenine dinucleotide phosphate
-
- NCBI
-
- National Center for Biotechnology Information
-
- NCE
-
- normochromatic erythrocytes
-
- NFI
-
- National Food Institute
-
- NIH
-
- Department of the National Institutes of Health
-
- NLM
-
- National Library of Medicine
-
- NOAEL
-
- no-observed-adverse-effect level
-
- NRL
-
- national reference laboratory
-
- P
-
- positive result
-
- p.o.
-
- per os (orally)
-
- PBMC
-
- peripheral blood mononuclear cells
-
- PbO2
-
- lead oxide
-
- PCE
-
- polychromated erythrocytes
-
- PCNA
-
- proliferating cell nuclear antigen
-
- QuEChERS
-
- Quick Easy Cheap Effective Rugged Safe
-
- RASFF
-
- Rapid Alert System for Food and Feed
-
- ROS
-
- reactive oxygen species
-
- RPA
-
- reference point for action
-
- SD
-
- standard deviation
-
- SHE
-
- Syrian hamster embryo
-
- SOD
-
- superoxide dismutase
-
- SPE
-
- solid-phase extraction
-
- SWG
-
- Standing Working Group
-
- T
-
- thymine
-
- T3
-
- triiodothyronine
-
- T4
-
- thyroxine
-
- TBLOQ
-
- toxicologically based limit of quantification
-
- TOF-MS
-
- time-of-flight mass spectrometry
-
- TPO
-
- thyroid peroxidase
-
- TrxR
-
- thioredoxin reductase
-
- TSH
-
- thyroid-stimulating hormone
-
- TTC
-
- threshold of toxicological concern
-
- UPLC
-
- ultra-performance liquid chromatography
-
- UV
-
- ultraviolet
-
- UV/Vis
-
- ultraviolet/visible
-
- VMP
-
- Veterinary medicinal product
-
- WHO
-
- World Health Organization
Appendix A – Identification and selection of evidence relevant for the risk assessment of malachite green and leucomalachite green in food
A.1 Search for scientific literature
A.1.1 Chemistry and methods of analysis
A search for recent reviews was conducted to identify papers on chemistry and methods of analysis by using the following search string in Web of Science and PubMed:
Used search string: TOPIC: ((“malachite green” OR “leucomalachite green”)) AND TOPIC: ((chemistry OR analysis OR determination OR detection OR ELISA OR immune* OR GC OR GC-MS OR HPLC OR LC-MS OR ICP-MS)) AND TOPIC: review; Timespan=last five years
From this search, four reviews were identified:
Liu MingYang; Xiao ShanShan; Yu Bing; Yang ChunGuang; Zhao JonhHong; Jin Yan; Zhu ChengYun, 2015. Research progress of the residue determination of triphenymethane dyes in aquatic product. Journal of Food Safety and Quality Volume: 6 Issue: 1 Pages: 35–40.
Hidayah, N.; Abu Bakar, F.; Mahyudin, N. A.; Faridah S; Nur-Azura M.S.; Zaman M.Z., 2013. Detection of malachite green and leuco-malachite green in fishery industry. International Food Research Journal Volume: 20 Issue: 4 Pages: 1511–1519 Published: 2013
Lopez-Gutierrez, Noelia; Romero-Gonzalez, Roberto; Martinez Vidal, Jose Luis; Garrida-Frenich Antonia, 2013. Analysis of triphenylmethane dyes in seafood products: a review of extraction methods and determination by liquid chromatography coupled to mass spectrometry. ANALYTICAL METHODS Volume: 5 Issue: 14 Pages: 3434–3449 Published: 2013.
Hashimoto, Juliana Campos; Rizzato Paschoal, Jonas Augusto; De Queiroz, Julio Ferraz; Reyes Reyes, Felix Guillermo, 2011. Considerations on the Use of Malachite Green in Aquaculture and Analytical Aspects of Determining the Residues in Fish: A Review. JOURNAL OF AQUATIC FOOD PRODUCT TECHNOLOGY Volume: 20 Issue: 3 Pages: 273–294 Published: 2011
These four reviews were used to identify papers on chemistry and methods of analysis. In addition, a literature search was conducted to identify the most recent papers published since 2012, as described below.
A.1.1.1 Web of Science
Used search string: TOPIC: (“malachite green” OR “leucomalachite green”) AND TOPIC: (chemistry OR analysis OR determination OR detection OR ELISA OR immune* OR GC OR GC-MS OR HPLC OR LC-MS OR ICP-MS) NOT TOPIC: (“waste water*” OR waste OR wastewater* OR adsorp* OR degradat* OR decoloriz* OR Ichthyophthirius OR mycobacterium OR tile OR ceramic OR pottery OR Saprolegnia OR salmonella OR coli); Refined by: RESEARCH AREAS: (CHEMISTRY OR FOOD SCIENCE TECHNOLOGY OR SPECTROSCOPY OR INSTRUMENTS INSTRUMENTATION OR AGRICULTURE OR VETERINARY SCIENCES OR FISHERIES); Timespan=2012-2015; Search language=Auto
Result in Web of Science: 267
A.1.1.2 PubMed
Used search string: (((((“malachite green” OR “leucomalachite green”)) AND (chemistry OR analysis OR determination OR detection OR elisa OR immune* OR GC OR gc-ms OR hplc OR lc-ms OR icp-ms)) AND (“2012/01/01”[PDAT]: “3000”[PDAT]))) NOT (“waste water*” OR waste OR wastewater* OR adsorp* OR degradati* OR decoloriz* OR ichthyophthirius OR mycobacterium OR tile OR ceramic OR potter OR saprolegnia OR salmonella OR coli)
Results in PubMed: 251
A.1.2 Occurrence and exposure
A search in Web of Science and PubMed was conducted to identify papers on occurrence and exposure by using the following search strings:
A.1.2.1 Web of Science
Used search string: TOPIC: (“malachite green” OR “leucomalachite green”) AND TOPIC: (occurrence OR exposure OR assessment OR levels OR concentrate* OR beverage OR food OR vegetable* OR fruit* OR grain OR cereal OR poultry OR meat OR eggs OR milk OR seafood OR fish OR shrimp OR prawns OR crustacean) NOT TOPIC: (“waste water” OR waste OR wastewater OR adsorp* OR degradati* OR decoloriz* OR Ichthyophthirius OR mycobacterium OR tile OR ceramic OR pottery OR Saprolegnia OR salmonella OR coli); Refined by: RESEARCH AREAS: (CHEMISTRY OR FOOD SCIENCE TECHNOLOGY OR VETERINARY SCIENCES OR FISHERIES OR SPECTROSCOPY OR INSTRUMENTS INSTRUMENTATION OR AGRICULTURE); Timespan=2002-2015;Search language=Auto
Result in Web of Science: 475
A.1.2.2 PubMed
Used search string: ((((“malachite green” OR “leucomalachite green”)) AND (occurrence OR exposure OR assessment OR levels OR concentrate* OR beverage OR food OR vegetable* OR fruit* OR grain OR cereal OR poultry OR meat OR eggs OR milk OR seafood OR fish OR shrimp OR prawns OR crustacean)) AND (“2002/01/01”[Date - Publication]: “3000”[Date - Publication])) NOT (“waste water” OR waste OR wastewater OR adsorp* OR degradati* OR decoloriz* OR ichthyophthirius OR mycobacterium OR tile OR ceramic OR potter OR saprolegnia OR salmonella OR coli OR soil)
Results in PubMed: 166
A.1.3 Occurrence due to illegal use as a food colouring agent
A search in Web of Science and PubMed was conducted to identify papers regarding the occurrence due to the illegal use of MG as a food colouring agent by using the following search strings:
A.1.3.1 Web of Science
Used search string: TOPIC: (“malachite green”) AND TOPIC: ((adulteration or “food colour*” or “food color*”)); Timespan=2002-2015; Search language=Auto
Result in Web of Science: 13
A.1.3.2 PubMed
Used search string: ((“malachite green”) AND (adulteration or “food colour*” or “food color*”)) AND (“2002/01/01”[Date - Publication]: “3000”[Date - Publication])
Results in PubMed: 2
A.1.4 Processing
A search in Web of Science and PubMed was conducted to identify papers on processing by using the following search strings:
A.1.4.1 Web of Science
Used search string: TOPIC: (“malachite green” OR “leucomalachite green”) AND TOPIC: (cooking OR roasting OR frying OR boiling OR baking OR “thermal processing”) NOT TOPIC: (“waste water” OR waste OR wastewater OR decolor* OR biodegrad* OR photocatal* OR photodegrad* OR adsorp* OR Ichthyophthirius OR mycobacterium OR tile OR ceramic OR pottery OR Saprolegnia OR salmonella OR coli); Refined by: RESEARCH AREAS: (CHEMISTRY OR VETERINARY SCIENCES OR AGRICULTURE OR FOOD SCIENCE TECHNOLOGY OR FISHERIES); Timespan=All years; Search language=Auto
Result in Web of Science: 29
A.1.4.2 PubMed
Used search string: (((“malachite green” OR “leucomalachite green”)) AND (processing OR cooking OR roasting OR frying OR boiling OR baking OR “thermal processing” OR sterilization))
Results in PubMed: 16
A.1.5 Metabolism and kinetics
A search in Web of Science and PubMed was conducted to identify papers on metabolism and kinetics by using the following search strings:
A.1.5.1 Web of Science
Used search string: TOPIC: (“malachite green” OR “leucomalachite green”) AND TOPIC: (toxicokinetic* OR metabolism* OR distribution OR excretion OR “mode of action” OR absorption OR biomarker OR biotransformation) NOT TOPIC: (“waste water*” OR fluorogen OR waste OR “surface plasmon resonance” OR SPR OR “light scattering” OR film* OR wastewater* OR soil* OR humic OR humus OR nano* OR decolor* OR biodegrad* OR photocatal* OR photodegrad* OR adsorp* OR Ichthyophthirius OR mycobacterium OR tile OR ceramic OR pottery OR Saprolegnia OR salmonella OR coli); Refined by: RESEARCH AREAS: (CHEMISTRY OR BIOCHEMISTRY MOLECULAR BIOLOGY OR PHARMACOLOGY PHARMACY OR TOXICOLOGY OR CELL BIOLOGY OR ENDOCRINOLOGY METABOLISM OR VETERINARY SCIENCES); Timespan=All years; Search language=Auto
Result in Web of Science: 557
A.1.5.2 PubMed
Used search string: (((“malachite green” OR “leucomalachite green”)) AND (toxicokinetic* OR metabolism* OR distribution OR excretion OR “mode of action” OR absorption OR biomarker OR biotransformation)) NOT (“waste water*” OR fluorogenic OR waste OR “surface plasmon resonance” OR SPR OR “light scattering” OR film* OR wastewater* OR soil* OR humic OR humus OR nano* OR decolor* OR biodegrad* OR photocatal* OR photodegrad* OR adsorp* OR ichthyophthirius OR mycobacterium OR tile OR ceramic OR potter OR saprolegnia OR salmonella OR coli)
Result in PubMed: 301
A.1.6 Toxicity
A search in Web of Science and PubMed was conducted to identify papers on toxicity by using the following search strings:
A.1.6.1 Web of Science
Used search string: TOPIC: (“malachite green” OR “leucomalachite green”) AND TOPIC: (toxicity OR toxi* OR mutagen* OR teratogen* OR carcinogen* OR carcino* OR genotox* OR reprotox* OR nephrotox* OR neurotox* OR hepatotox* OR immunotox* OR haemotox* OR hematotox* OR cytotox* OR “develop* toxicity” OR endocri*) NOT TOPIC: (“waste water*” OR fluorogen OR waste OR “surface plasmon resonance” OR SPR OR “light scattering” OR film* OR wastewater* OR soil* OR humic OR humus OR nano* OR decolor* OR biodegrad* OR photocatal* OR photodegrad* OR adsorp* OR Ichthyophthirius OR mycobacterium OR tile OR ceramic OR pottery OR Saprolegnia); Refined by: RESEARCH AREAS: (TOXICOLOGY OR CARDIOVASCULAR SYSTEM CARDIOLOGY OR ANATOMY MORPHOLOGY OR PHARMACOLOGY PHARMACY OR OPHTHALMOLOGY OR BIOCHEMISTRY MOLECULAR BIOLOGY OR LIFE SCIENCES BIOMEDICINE OTHER TOPICS OR DERMATOLOGY OR HEMATOLOGY OR GERIATRICS GERONTOLOGY OR ONCOLOGY OR UROLOGY NEPHROLOGY OR CELL BIOLOGY OR PATHOLOGY OR RHEUMATOLOGY OR ENDOCRINOLOGY METABOLISM OR RESPIRATORY SYSTEM OR ORTHOPEDICS OR OBSTETRICS GYNECOLOGY OR NEUROSCIENCES NEUROLOGY OR DEVELOPMENTAL BIOLOGY OR GASTROENTEROLOGY HEPATOLOGY OR DENTISTRY ORAL SURGERY MEDICINE OR IMMUNOLOGY OR REPRODUCTIVE BIOLOGY OR ALLERGY OR PEDIATRICS OR PUBLIC ENVIRONMENTAL OCCUPATIONAL HEALTH); Timespan=All years; Search language=Auto
Result in Web of Science: 555
A.1.6.2 PubMed
Used search string: (((((“malachite green” OR “leucomalachite green”)) AND (toxicity OR toxi* OR mutagen* OR teratogen* OR carcinogen* OR carcino* OR genotox* OR reprotox* OR nephrotox* OR neurotox* OR hepatotox* OR immunotox* OR haemotox* OR hematotox* OR cytotox* OR “develop* toxicity” OR endocri*))) NOT (“waste water*” OR fluorogen* OR waste OR film* OR wastewater* OR soil* OR humic OR humus OR nano* OR decolor* OR biodegrad* OR photocatal* OR photodegrad* OR adsorp* OR Ichthyophthirius OR mycobacterium OR tile OR ceramic OR pottery OR Saprolegnia))
Result in PubMed: 132
A.1.7 Human studies
A search in Web of Science and PubMed was conducted to identify papers on human data by using the following search strings.
A.1.7.1 Web of Science
Used search string: TOPIC: (“malachite green” OR “leucomalachite green”) AND TOPIC: (epidemiology OR biomarker OR cohort OR “case control” OR “case stud*” OR “adverse effect*”) NOT TOPIC: (“waste water*” OR fluorogen OR waste OR “surface plasmon resonance” OR SPR OR “light scattering” OR film* OR wastewater* OR soil* OR humic OR humus OR nano* OR decolor* OR biodegrad* OR photocatal* OR photodegrad* OR adsorp* OR Ichthyophthirius OR mycobacterium OR tile OR ceramic OR pottery OR Saprolegnia); Refined by: RESEARCH AREAS: (TOXICOLOGY OR HEMATOLOGY OR PHARMACOLOGY PHARMACY OR DEVELOPMENTAL BIOLOGY OR ANATOMY MORPHOLOGY OR UROLOGY NEPHROLOGY OR SURGERY OR PEDIATRICS OR RHEUMATOLOGY OR BIOCHEMISTRY MOLECULAR BIOLOGY OR IMMUNOLOGY OR RESPIRATORY SYSTEM OR PUBLIC ENVIRONMENTAL OCCUPATIONAL HEALTH OR DERMATOLOGY OR RESEARCH EXPERIMENTAL MEDICINE OR CARDIOVASCULAR SYSTEM CARDIOLOGY OR REPRODUCTIVE BIOLOGY OR OPHTHALMOLOGY OR ONCOLOGY OR PATHOLOGY OR GERIATRICS GERONTOLOGY OR GASTROENTEROLOGY HEPATOLOGY OR CELL BIOLOGY OR ANESTHESIOLOGY OR DENTISTRY ORAL SURGERY MEDICINE OR ENDOCRINOLOGY METABOLISM); Timespan=All years; Search language=Auto
Result in Web of Science: 63
A.1.7.2 PubMed
Used search string: (((“malachite green” OR “leucomalachite green”)) AND (epidemiology OR biomarker OR cohort OR “case control” OR “case stud*” OR “adverse effect*”)) AND (“waste water*” OR fluorogen* OR waste OR film* OR wastewater* OR soil* OR humic OR humus OR nano* OR decolor* OR biodegrad* OR photocatal* OR photodegrad* OR adsorp* OR Ichthyophthirius OR mycobacterium OR tile OR ceramic OR pottery OR Saprolegnia)
Result in PubMed: 11
A.2 Exclusion criteria for abstracts
- Papers related to the use as dye (e.g. colouring of plastics or clothes)
- Papers related to environmental science (e.g. wastewater treatment)
- Papers related to ornamental plants/plant varieties/plant health
- Papers related to the efficacy of the drug/treatment of infections
- Papers related to feed (occurrence and analytical methods)
- Papers related to the use of MG for the detection of other compounds/spectrophotometric characteristics of MG
- Toxicity studies in non-mammalian animals, except for studies related to the mode of action and toxicokinetics
- Papers related to microbiological methods and general microbiology
- Case reports on pets.
A.3 EFSA guidance documents applied for the risk assessment
- EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA Journal 2005;3(10):282, 31 pp. doi:10.2903/j.efsa.2005.282
- EFSA (European Food Safety Authority), 2006. Guidance of the Scientific Committee on a request from EFSA related to uncertainties in Dietary Exposure Assessment. EFSA Journal 2006;4(12):438, 54 pp. doi:10.2903/j.efsa.2007.438
- EFSA (European Food Safety Authority), 2009a. Guidance of the Scientific Committee on use of the benchmark dose approach in risk assessment. EFSA Journal 2009;7(6):1150, 72 pp. doi:10.2903/j.efsa.2009.1150
- EFSA (European Food Safety Authority), 2009b. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: General principles. EFSA Journal 2009;7(5):1051, 22 pp. doi:10.2903/j.efsa.2009.1051
- EFSA (European Food Safety Authority), 2010. Management of left-censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. doi:10.2903/j.efsa.2010.1557
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. doi:10.2903/j.efsa.2011.2097
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal;9(3):1970, 22 pp. doi:10.2903/j.efsa.2011.1970
- EFSA (European Food Safety Authority), 2011c. Report on the development of a food classification and description system for exposure assessment and guidance on its implementation and use. EFSA Journal;9(12):2489, 438 pp. doi:10.2903/j.efsa.2011.2489
- EFSA (European Food Safety Authority), 2011d. Use of BMDS and PROAST software packages by EFSA Scientific Panels and Units for applying the Benchmark Dose (BMD) approach risk assessment. Technical Report. EFSA Supporting Publications 2011, EN-113, 190 pp.
- EFSA (European Food Safety Authority), 2011e. Overview of the procedures currently used at EFSA for the assessment of dietary exposure to different chemical substances. EFSA Journal 2011;9(12):2490, 33 pp. doi:10.2903/j.efsa.2011.2490
- EFSA Scientific Committee, 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. doi:10.2903/j.efsa.2011.2379
- EFSA Scientific Committee, 2012a. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. doi:10.2903/j.efsa.2012.2579
- EFSA Scientific Committee, 2012b. Scientific Opinion on Risk Assessment Terminology. EFSA Journal 2012;10(5):2664, 43 pp. doi:10.2903/j.efsa.2012.2664
Appendix B – Dietary surveys used for the estimation of chronic dietary exposure to malachite green at the reference point for action
| Country | Survey acronymc | Survey period | Number of days per subject | Number of subjectsa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Infants | Toddlers | Other children | Adolescents | Adults | Elderly | Very elderly | ||||
| Austria | ASNS – Adults | 2010–2012 | 2 | – | – | – | – | 308 | 67 | 25 |
| Austria | ASNS – Children | 2010–2012 | 3 | – | – | 128 | 237 | – | – | – |
| Belgium | Regional Flanders | 2002–2002 | 3 | – | 36b | 625 | – | – | – | – |
| Belgium | Diet National 2004 | 2004 | 2 | – | – | – | 576 | 1,292 | 511 | 704 |
| Bulgaria | NUTRICHILD | 2007 | 2 | 859 | 428 | 433 | – | – | – | – |
| Cyprus | Childhealth | 2003 | 3 | – | – | – | 303 | – | – | – |
| Czech Republic | SISP04 | 2003–2004 | 2 | – | – | 389 | 298 | 1,666 | – | – |
| Denmark | DANSDA 2005-08 | 2005–2008 | 7 | – | – | 298 | 377 | 1,739 | 274 | 12b |
| Denmark | IAT 2006 07 | 2006–2007 | 7 | 826 | 917 | – | – | – | – | – |
| Finland | DIPP 2001 2009 | 2001–2009 | 3 | 500 | 500 | 750 | – | – | – | – |
| Finland | NWSSP07 08 | 2007–2008 | 4 | – | – | – | 306 | – | – | – |
| Finland | FINDIET2012 | 2012 | 2 | – | – | – | – | 1,295 | 413 | – |
| France | INCA2 | 2007 | 7 | – | – | 482 | 973 | 2,276 | 264 | 84 |
| Germany | VELS | 2001–2002 | 6 | 159 | 348 | 293 | – | – | – | – |
| Germany | EsKiMo | 2006 | 3 | – | – | 835 | 393 | – | – | – |
| Germany | National Nutrition Survey II | 2007 | 2 | – | – | – | 1,011 | 10,419 | 2,006 | 490 |
| Greece | Regional Crete | 2004–2005 | 3 | – | – | 838 | – | – | – | – |
| Greece | DIET LACTATION GR | 2005–2007 | 3 | – | – | – | – | 49b | – | – |
| Hungary | National Repr Surv | 2003 | 3 | – | – | – | – | 1,074 | 206 | 80 |
| Ireland | NANS 2012 | 2008–2010 | 4 | – | – | – | – | 1,274 | 149 | 77 |
| Italy | INRAN SCAI 2005 06 | 2005–2006 | 3 | 16b | 36b | 193 | 247 | 2,313 | 290 | 228 |
| Latvia | EFSA TEST | 2008 | 2 | – | – | 187 | 453 | 1,271 | – | – |
| Latvia | FC PREGNANTWOMEN 2011 | 2011 | 2 | – | – | – | – | 1,002 | – | – |
| Netherlands | VCP kids | 2006–2007 | 3 | – | 322 | 957 | – | – | – | – |
| Netherlands | VCPBasis AVL2007 2010 | 2007–2010 | 2 | – | – | 447 | 1,142 | 2,057 | 173 | – |
| Netherlands | VCP-Elderly | 2010–2012 | 2 | – | – | – | – | – | 289 | 450 |
| Romania | Dieta Pilot Adults | 2012 | 7 | – | – | – | – | 1,254 | 83 | 45b |
| Spain | enKid | 1998–2000 | 2 | – | 17b | 156 | 209 | – | – | – |
| Spain | AESAN | 1999–2001 | 3 | – | – | – | – | 410 | – | – |
| Spain | NUT INK05 | 2004–2005 | 2 | – | – | 399 | 651 | – | – | – |
| Spain | AESAN FIAB | 2009 | 3 | – | – | – | 86 | 981 | – | – |
| Sweden | NFA | 2003 | 4 | – | – | 1,473 | 1,018 | – | – | – |
| Sweden | Riksmaten 2010 | 2010–2011 | 4 | – | – | – | – | 1,430 | 295 | 72 |
| UK | NDNS-RollingProgrammeYears1-3 | 2008–2011 | 4 | – | 185 | 651 | 666 | 1,266 | 166 | 139 |
| UK | DNSIYC 2011 | 2011 | 4 | 1,369 | 1,314 | – | – | – | – | – |
- a Subjects who reported at least 2 days of food consumption data.
- b 95th percentiles calculated over a number of observations fewer than 60. These require cautious interpretation, as the results may not be statistically robust (EFSA, 2011b).
- c More information on the dietary surveys is given in the EFSA guidance ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011b).
Appendix C – Benchmark dose analysis
- the incidence of hepatocellular adenomas and carcinomas in female B6C3F1 mice exposed to leucomalachite green (LMG) in feed for 2 years (NTP, 2005; Culp et al., 2006) (see Table C.1);
- increased absolute liver weight in female F344 rats exposed to malachite green (MG) in feed for 28 days (Culp et al., 1999; NTP, 2004) (see Table C.3);
- increased absolute liver weight in male F344 rats exposed to LMG in feed for 2 years (NTP, 2005; Culp et al., 2006) (see Table C.6);
- decreased body weight in male and female F344 rats exposed to MG or LMG in feed for 2 years (NTP, 2005; Culp et al., 2006) (see Table C.9).
For quantal endpoints, the BMD/L values were calculated by means of the software BMDS v.2.4. All models for dichotomous (quantal) data available were selected for the BMD analysis using the default benchmark response (BMR) of 10% extra risk as advised by the EFSA guidance on the use of benchmark dose (EFSA, 2009b). In the first instance, all models were run without restrictions. Acceptance of a model (and its benchmark dose lower confidence limit (BMDL)) was defined through the log-likelihood value and its goodness-of-fit test with a p > 0.05. The lowest BMDL of all accepted models fitted to the data of one endpoint and one dose–response data set was determined as the BMDL of that data set.
For continuous endpoints, the BMD/L values were calculated by means of the software PROAST (v26). The exponential and Hill models were selected for the BMD analysis using the default benchmark response (BMR) of 5% extra risk as advised by the EFSA guidance on the use of benchmark dose (EFSA, 2009b). Acceptance of a model (and its BMDL) was defined through the log-likelihood value and its goodness-of-fit test with a p > 0.05. The lowest BMDL of all accepted models fitted to the data of one endpoint and one dose–response data set, was determined as the BMDL of that data set.
C.1 Hepatocellular adenomas and carcinomas in female B6C3F1 mice exposed to leucomalachite green
The incidences of hepatocellular adenomas and carcinomas in female mice exposed to LMG are shown in Table C.1.
The results of the BMD analysis are shown in Table C.2. The CONTAM Panel noted the small (non-significant) difference between the log-likelihood values for the null and full model, indicating that both models do not differ significantly. The CONTAM Panel noted the very large BMD/BMDL ratio for the gamma model and the disparity between the lower 95% confidence limit for a benchmark response of 10% extra risk (BMDL10) obtained for the gamma model and the BMDL10 values obtained from other models. Therefore, the gamma model was not considered further. It was also noted that no BMDL10 was calculated for several models. Based on these observations, restricted models were also evaluated (see Table C.2).
From this BMD analysis, a lowest BMDL10 of 13.1 mg/kg body weight (bw) per day was identified (Table C.2, Figure C.1).
| Dose reported by NTP (mg/kg bw per day) | Number of animals | Number of animals with hepatocellular adenoma or carcinoma |
|---|---|---|
| 0 | 47 | 3 |
| 13 | 48 | 6 |
| 31 | 47 | 6 |
| 63 | 47 | 11 |
- NTP: National Toxicology Program; bw: body weight.
| Models | Restriction | Number of parameters | Log-likelihood | p-Value | Accepted | BMD10 (mg/kg bw per day) | BMDL10 (mg/kg bw per day) |
|---|---|---|---|---|---|---|---|
| Full | n.a. | 4 | −72.77 | – | – | – | – |
| Null | n.a. | 1 | −75.70 | – | – | – | – |
| Gamma | None | 3 | −72.94 | 0.5586 | Yes | 34.6 | 9.5E-43 |
| Logistic | n.a. | 2 | −72.97 | 0.8138 | Yes | 43.1 | 31.2 |
| LogLogistic | None | 3 | −72.94 | 0.5492 | Yes | 34.6 | n.c. |
| LogProbit | None | 3 | −72.97 | 0.522 | Yes | 33.5 | n.c. |
| Multistage | None | 3 | −72.93 | 0.5611 | Yes | 36.8 | 13.1 |
| Multistage-Cancer | n.a. | 3 | −72.93 | 0.5611 | Yes | 36.8 | 20.1 |
| Probit | n.a. | 2 | −72.96 | 0.8223 | Yes | 41.9 | 29.5 |
| Weibull | None | 3 | −72.94 | 0.558 | Yes | 34.8 | n.c. |
| Quantal-Linear | n.a. | 2 | −72.94 | 0.8417 | Yes | 35.4 | 20.1 |
| Gamma | Default | 2 | −72.94 | 0.8417 | Yes | 35.4 | 20.1 |
| LogLogistic | Default | 3 | −72.94 | 0.5492 | Yes | 34.6 | 18.5 |
| LogProbit | Default | 2 | −73.18 | 0.6589 | Yes | 46.5 | 30.4 |
| Multistage | Default | 3 | −72.93 | 0.5611 | Yes | 36.8 | 20.1 |
| Weibull | Default | 2 | −72.94 | 0.8417 | Yes | 35.4 | 20.1 |
- BMD10: benchmark dose for a benchmark response of 10% extra risk; BMDL10: the lower 95% confidence limit for a benchmark response of 10% extra risk; n.a.: not applicable; n.c.: not calculated by BMDS software.
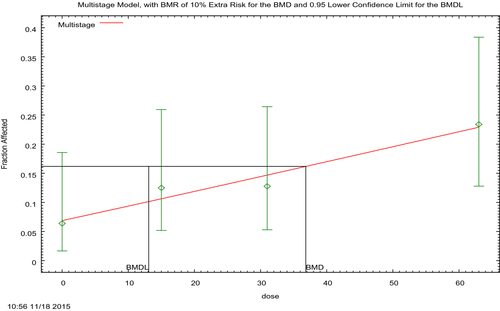
C.2 Increased absolute liver weight in female F344 rats exposed to malachite green in a 28-day study
The data on increased liver weight in female rats exposed to MG are shown in Table C.3. The results of the BMD analysis are shown in Tables C.4 and C.5 and in Figure C.2.
From this BMD analysis, a lowest BMDL05 of 5.9 mg/kg bw per day was identified (Table C.4, Figure C.2).
| Dose reported by NTP (mg/kg bw per day) | Number of animals | Liver weight (g) | Standard deviation |
|---|---|---|---|
| 0 | 8 | 4.257 | 0.104 |
| 3 | 8 | 4.491 | 0.233 |
| 12 | 8 | 4.457 | 0.221 |
| 40 | 8 | 4.924 | 0.148 |
| 75 | 8 | 4.996 | 0.089 |
| 190 | 8 | 4.953 | 0.116 |
- NTP: National Toxicology Program; bw: body weight.
| Model | BMDL05 (mg/kg bw per day) | BMD05 (mg/kg bw per day) | BMDU05 (mg/kg bw per day) |
|---|---|---|---|
| Exponential | 5.9 | 9.1 | 13.9 |
| Hill | 11.4 | 17.1 | 33.7 |
- bw: body weight; BMD05: benchmark dose for a response of 5% extra risk; BMDL05: lower 95% confidence limit for a benchmark response of 5% extra risk; BMDU05: upper 95% confidence limit for a benchmark response of 5% extra risk.
| Exponential model a | |||
|---|---|---|---|
| Model | Converged | npar | loglik |
| Full | 1 | 7 | 95.50 |
| m1- | 1 | 2 | 59.18 |
| m2- | 1 | 3 | 71.39 |
| m3- | 1 | 4 | 85.25 |
| m4- | 1 | 4 | 90.20 |
| m5- | 1 | 5 | 91.01 |
| m4- | 1 | 4 | 90.20 |
| Hill model b | |||
|---|---|---|---|
| Model | Converged | npar | loglik |
| Full | 7 | 95.50 | |
| m1- | 1 | 2 | 59.18 |
| m2- | 1 | 3 | 70.94 |
| m3- | 1 | 4 | 85.10 |
| m4- | 1 | 4 | 88.61 |
| m5- | 1 | 5 | 90.82 |
| m4- | 1 | 5 | 90.82 |
- a The chosen exponential model was m4-.
- b The chosen Hill model was m5-.
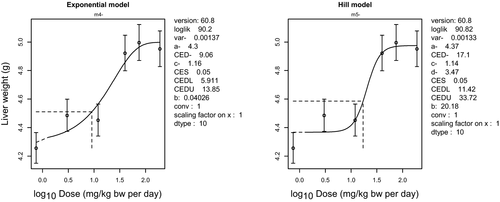
C.3 Increased absolute liver weight in male F344 rats exposed to leucomalachite green in a long-term study
The data on increased liver weight in male rats exposed to LMG are shown in Table C.6. The results of the BMD analysis are shown in Tables C.7 and C.8, and in Figure C.3.
From this BMD analysis, a lowest BMDL05 of 7.2 mg/kg bw per day was identified (Table C.7, Figure C.3).
| Dose reported by NTP (mg/kg bw per day) | Number of animals | Liver weight (g) | Standard deviation |
|---|---|---|---|
| 0 | 23 | 14.73 | 0.52 |
| 5 | 29 | 14.81 | 0.69 |
| 15 | 34 | 17.18 | 0.67 |
| 30 | 29 | 19.29 | 0.69 |
- NTP: National Toxicology Program; bw: body weight.
| Model | BMDL05 (mg/kg bw per day) | BMD05 (mg/kg bw per day) | BMDU05 (mg/kg bw per day) |
|---|---|---|---|
| Exponential | 7.3 | 9.9 | 12.9 |
| Hill | 7.2 | 9.5 | 12.6 |
- bw: body weight; BMD05: benchmark dose for a response of 5% extra risk; BMDL05: lower 95% confidence limit for a benchmark response of 5% extra risk; BMDU05: upper 95% confidence limit for a benchmark response of 5% extra risk.
| Exponential model a | |||
|---|---|---|---|
| Model | Converged | npar | loglik |
| Full | 1 | 5 | 210.18 |
| m1- | 1 | 2 | 83.56 |
| m2- | 1 | 3 | 196.14 |
| m3- | 1 | 4 | 196.15 |
| m4- | 0 | 4 | 196.89 |
| m5- | 1 | 5 | 210.18 |
| m5- | 1 | 5 | 210.18 |
| Hill model b | |||
|---|---|---|---|
| Model | Converged | npar | loglik |
| Full | 5 | 210.18 | |
| m1- | 1 | 2 | 83.56 |
| m2- | 1 | 3 | 193.95 |
| m3- | 1 | 4 | 194.66 |
| m4- | 0 | 4 | 196.81 |
| m5- | 1 | 5 | 210.18 |
| m5- | 1 | 5 | 210.18 |
- a The chosen exponential model was m5-.
- b The chosen Hill model was m5-.
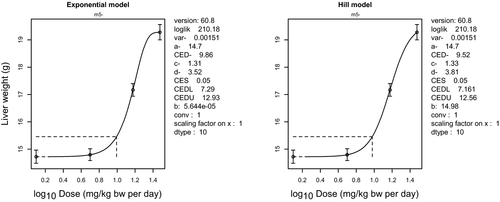
C.4 Decreased body weight in male and female F344 rats exposed to leucomalachite green or malachite green in a long-term study
Both MG and LMG caused a decreased body weight in rats and the data are shown in Table C.9. MG was only tested in male rats while LMG was tested in both sexes. The data from both compounds and both sexes were combined for BMD analysis, using substance and sex as covariate. As MG and LMG have a different molecular weight, the doses were expressed as mmol/kg bw per day for the BMD analysis. The results from the BMD analysis were then converted to mg LMG/kg bw per day. Table C.10 shows the BMD(L/U) values both expressed as mmol/kg bw per day and mg LMG/kg bw per day.
The results of the BMD analysis are shown in Tables C.10 and C.11 and in Figure C.4.
From this BMD analysis, a lowest BMDL05 of 5.8 mg/kg bw per day was identified (Table C.10, Figure C.4).
| Substance | Dose (mg/kg bw per day) | Dose (mmol/kg bw per day) | Number of animals | Sex | Body weight (g) | Standard deviation |
|---|---|---|---|---|---|---|
| MG | ||||||
| 0 | 0 | 28 | F | 315 | 7 | |
| 7 | 0.0192 | 23 | F | 312 | 10 | |
| 21 | 0.0575 | 32 | F | 287 | 9 | |
| 43 | 0.1178 | 25 | F | 277 | 10 | |
| LMG | ||||||
| 0 | 0 | 38 | F | 312 | 7 | |
| 6 | 0.0182 | 36 | F | 299 | 7 | |
| 17 | 0.0514 | 35 | F | 277 | 7 | |
| 35 | 0.1059 | 33 | F | 240 | 7 | |
| 0 | 0 | 23 | M | 432 | 8 | |
| 5 | 0.0151 | 29 | M | 424 | 10 | |
| 15 | 0.0454 | 34 | M | 397 | 10 | |
| 30 | 0.0908 | 29 | M | 377 | 10 | |
- bw: body weight; LMG : leucomalachite green: MG: malachite green.
| Values expressed as mmol/kg bw per day | ||||
|---|---|---|---|---|
| Model | BMDL05 | BMD05 | BMDU05 | |
| LMG female | ||||
| Exponential | 0.01766 | 0.0201 | 0.0227 | |
| Hill | 0.01794 | 0.0204 | 0.02298 | |
| LMG male | ||||
| Exponential | 0.02903 | 0.0325 | 0.03627 | |
| Hill | 0.02992 | 0.0335 | 0.03720 | |
| MG female | ||||
| Exponential | 0.03753 | 0.0429 | 0.04899 | |
| Hill | 0.03852 | 0.044 | 0.05018 | |
| Values expressed as mg LMG/kg bw per day | ||||
|---|---|---|---|---|
| Model | BMDL05 | BMD05 | BMDU05 | |
| LMG female | ||||
| Exponential | 5.8 | 6.6 | 7.5 | |
| Hill | 5.9 | 6.7 | 7.6 | |
| LMG male | ||||
| Exponential | 9.6 | 10.7 | 12.0 | |
| Hill | 9.9 | 11.1 | 12.3 | |
| MG female | ||||
| Exponential | 13.7 | 15.7 | 17.9 | |
| Hill | 14.1 | 16.1 | 18.3 | |
- bw: body weight; BMD05: benchmark dose for a response of 5% extra risk; BMDL05: lower 95% confidence limit for a benchmark response of 5% extra risk; BMDU05: upper 95% confidence limit for a benchmark response of 5% extra risk; LMG: leucomalachite green: MG: malachite green.
| Exponential model a | |||
|---|---|---|---|
| Model | Converged | loglik | npar |
| Full | 1 | 813.21 | 13 |
| Full-v | 1 | 817.15 | 15 |
| m1-v | 1 | 224.34 | 4 |
| m2-v | 1 | 383.36 | 5 |
| m2-av | 1 | 707.75 | 7 |
| m2-bv | 1 | 553.01 | 7 |
| m2-abv | 1 | 796.37 | 9 |
| m3-abv | 1 | 796.45 | 10 |
| m4-abv | 1 | 796.37 | 10 |
| m5-abv | 1 | 796.45 | 11 |
| m5-bv | 1 | 525.41 | 9 |
| m5-av | 1 | 717.04 | 9 |
| m5-abv | 1 | 796.45 | 11 |
| Hill model b | |||
|---|---|---|---|
| Model | Converged | loglik | npar |
| Full-v | 817.15 | 15 | |
| m1-v | 1 | 224.34 | 4 |
| m2-v | 1 | 361.39 | 5 |
| m2-av | 1 | 709.98 | 7 |
| m2-bv | 0 | 523.71 | 7 |
| m2-abv | 1 | 794.53 | 9 |
| m3-abv | 1 | 795.28 | 10 |
| m4-abv | 1 | 794.53 | 10 |
| m5-abv | 1 | 795.28 | 11 |
| m5-bv | 1 | 524.36 | 9 |
| m5-av | 1 | 716.88 | 9 |
| m5-abv | 1 | 795.28 | 11 |
- a The chosen exponential model was m5-abv.
- b The chosen Hill model was m5-abv.
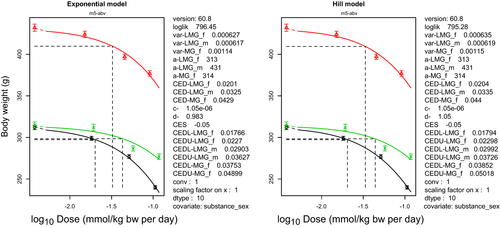
Dose–response data and fitted exponential and Hill model for the body weight in male (m) and female (f) rats exposed to malachite green (MG) or leucomalachite green (LMG) (dose expressed as mmol/kg bw per day) for a benchmark response of 5% (substrate and sex were analysed as covariate)
- The red line indicates male rats exposed to LMG, the green line female rats exposed to MG and the black line female rats exposed to LMG.